Cold Thermogenesis 7: ENVIRONMENT TRUMPS NUCLEAR GENOME
Readers Summary
- What shapes us, genes or environment?
- What is the normal circadian biology in a 24 hour day?
CT-7 is about how we are shaped by our environment by the evolutionary erosion of time that our ancestors faced. All life on this planet is shaped by two major variables in our environment: the sun and the seasonal changes. No matter the place present on earth, there are always alterations in these two factors that are cyclic, and always accounted for by all living organisms in some fashion. In some mammals, like man, it is accounted for centrally in the brain and peripherally in our organ ultradian clocks.
Today, we have proof this is true in the microbiome of man. Our nuclear genes are just not as important as our daily habits in determining our gut microbiome. Mitochondriac wisdom is getting more proof that environment controls our genes. I wonder when researchers will gain this insight? Environmental signals in waveforms affect our mt DNA and our mitochondrial matrix to modulate the function of the nuclear genes. LINK

This is why we have different patterns of aging in certain organs. From an evolutionary perspective, this makes a tremendous amount of sense because life is using the “knowns” of its environment to construct a reality that will ensure its survival. This is the basis of epigenetic signaling that we now know to be the major genetic modifier of the genome of all animals.Organisms do not have life cycles, rather they are life cycles because they are perpetual motion machines harboring subatomic particles that move and change their spin only when life is animated. When the particles stop moving and spinning in them they go from biotic to abiotic and light leaves them and information of organization is lost.
The major signal transducer in Epigenetics is found in the cellular signaling in our cell membranes that interact with the environment and our inner hormones that signal our epigenetic switches sitting on our genes inside the nucleus. Since it is clear that our cold adapted pathways use sensory afferents to signal to open the Ancient Pathway, I think it is time we just have a blog in the CT series that discusses what a normal 24 hour day is like in a human circadian biology.
We will start our day at 6 AM for the sake of the blog. Non Scientists just read only your parts of this blog the first time. We have lots of GEEKS reading this stuff now so I need to hurt their brains so they get the significance of what I am saying from an evolutionary perspective. Neurosurgery geeks have their own separate area because this post has lots of neuroanatomy in it. This should be a review of them and may make them fall asleep—though sleep is a very good thing for those geeks and, more generally, all of us.
How does our day begin and how does it evolve?
Non Scientists: This is the modern warm adapted human circadian cycle:
1. Our brain wakes up with a morning surge of cortisol. That is what turns our brain on at 6 AM. VIP helps do this in long light cycles. VIP is highest at 6 AM and lowest at 6 PM. Ghrelin is also highest in the morning. Ghrelin is an incretin hormone made in the stomach that has a half-life of one hour. NPY and Agouti stimulate the production of ghrelin. Ghrelin sends a signal directly to our pituitary gland and it influences our metabolism. This is why the circadian cycle in the stomach in the morning is critical to optimal health. I laid that out here in this blog and it is an important part of the Leptin Rx reset protocol.
Circadian cycles for the obese are dramatically altered compared to non-obese individuals in the morning. In the normal person, Ghrelin is high when cortisol is highest in the early morning. In them, ghrelin drops fast when food is eaten too. In the obese, ghrelin is much lower in the morning than expected. Moreover, when food is eaten, ghrelin stays elevated for an extended amount of time. This happens because of the inflammation associated with the higher leptin levels in the morning in the obese. Melatonin is known to acutely decrease ghrelin and sometimes in tough cases, I will use supplemental melatonin to demolish the morning ghrelin spikes in people with huge appetites. This is most common in the obese, eating disorders, and in those with a severe leaky gut who crave dairy and carbohydrates. It is also very common for young paleo enthusiasts because of how they embrace blue light technology gadgets of the modern world that destroy melatonin levels in the brain. Ghrelin spikes and stimulates NPY in the hypothalamus increasing our desire and ability to eat a lot more. Leptin makes NPY decline normally, but if one is leptin resistant this does not occur and appetite is out of control at the brain level.
This is why obesity is an inflammatory brain disorder causing hormonal imbalance. Hormone imbalance implies a poor redox potential in different parts of the body. Where the potential is destroyed a certain disease will manifest. Obesity happens when it occurs at the leptin receptor or due to slow energy leak from the inner mitochondrial membrane. This means the obese person is losing energy in black box radiation. It is easy to check but few do with a thermal camera. We see this macroscopically as major alteration is sweating and down-regulation of activity due to an inability to uncouple oxidative phosphorylation at the mitochondrial level. It is not a disease of stress or emotion as medicine is trying to ram down media outlets. It is a problem of an alters the quantum biology of electron/proton tunneling across our proteins.
Moreover, this should explain why the SAD breakfast is so problematic for modern humans. It is marketed as a carbohydrate fest. It is also why the Leptin Rx recommendation for protein and fat are so high in the morning. Protein is the backbone of all life. When we are losing energy and increasing molecular chaos we need to replace our proteins to recapture our balance. It solves this problem fast. I use protein over fat in the Leptin Rx because high-fat levels with low protein in the morning cause a spike in the gastric inhibitory peptide that can induce insulin resistance by itself. I do use high fat in certain cases, like bariatric surgery, eating disorders, hypothalamic amenorrhea, or high EMF levels. Many people do not know this. This is why so many people do not buy Gary Taubes theory of “Why We Get Fat”. Gary has only part of the story correct, in my view, because obesity occurs on a spectrum just like autism does because it depends on how the environment affects epigenetic expression. When you understand circadian biology, you get a much more complete picture of how the system works on a 24-hour basis. It turns out electrons control the coupling of biochemistry in life and understanding this helps to make sense of why hormones are disrupted when electrons are not handled correctly. I became a student of circadian biology when I saw the entire view from a 30,000-foot level.
2. At 6:45 AM we will expect to see the sharpest rise in blood pressure in the entire day. This is due to many activated systems in the body getting us ready to fully supply blood to all vital areas to get us motivated to begin our day and search for food. This period of rapid BP rise is why we see so many cardiac deaths occur in early morning sleep or early wakefulness. This happens when cortisol is highest.
3. At daybreak, when the sun hits the retina, the photic stimulus begins to shut off the secretion of melatonin from the pineal gland in the brain. AM sunlight contains mostly IR light at daybreak and as we approach noon, UV light frequencies appear on the skin.
4. At 7:30 AM usually after an hour of light melatonin is completely shut off in the brain.
5. At 8:30 the gut has been awakened and peristalsis becomes more vigorous and bowel movements getting rid of yesterday’s food are very likely. This happens by protons flows to move serotonin sulfated by the light of the gut microbiome in the wall to get to the brainstem to become sulfated melatonin. This is stimulated if food is eaten around this time as well. This is called the gastrocolic reflex. Cortisol, aldosterone, and ghrelin are all raised at this time to drive activity, increase our blood pressure and stimulate feeding. This is all yoked to AM sunlight stimulus. It is blocked when we wear clothes or at work in the AM.
6. Around 9-10 AM we have the highest secretions of the sex steroid hormones in humans and these pulsatile crescendos lead to our highest alertness at around 10 AM in our day to allow us to explore our environment.
7. Our ideal muscle coordination occurs at 2:30 PM and this adapts us best to hunt for dinner at this time. An hour later we see our fastest reaction times develop from our motor systems in our CNS.
8. At 5 PM humans exhibit their greatest cardiovascular efficiency allowing for maximal exercising or hunting. This also occurs during a period of time when we have our best rates of protein synthesis in our body. This is why exercise should be optimally done in this window.
9. As the sun falls at 6 PM we begin to see a major change in the cardiovascular system about a half hour later.
10. At 6:30 PM we see our highest blood pressures due to changes in atrial natriuretic factor and antidiuretic hormone (ANF, ADH) in the renin-aldosterone axis.
11. Once this occurs over the next 30 minutes (7 PM) we begin to see a gradual rise in our body temperature as leptin (and IL-6) is released from our fat stores, with agouti’s help, slowly after dinner is eaten to signal the brain about our fat mass and inflammatory status.
12. For the next two to three hours leptin levels slowly rise as insulin levels fall. Adiponectin levels also fall during this time frame. These fat hormone signals are what activate adenosine system in our bodies. Adenosine is created over the course of the day; high levels of adenosine lead to sleepiness.
13. This peaks at 10 PM and then the circadian clock allows for melatonin secretion after 3-4 hours of total darkness. Serum leptin is rising quickly now (with agouti’s help) as it is released from the fat cells to enter the brain. Agouti is highest at this time of the day, even in a normal person.
14. As these trends continue the GI tract is slowly shut down by the circadian clocks and around 11:30 PM and bowel movements are shut down for the night. This means that the vagus nerve is quiet.
15. At midnight leptin begins to enter the hypothalamus to bind to its receptor in the hypothalamus to signal energy reserves while also yoking energy metabolism to sleep via the hypocretin neurons that control all the sleep cycles. In diurnal animals, sleepiness occurs as the circadian element causes the release of the hormone melatonin and a gradual decrease in core body temperature. This drop in temperature is the stimulus to change sulfated serotonin to sulfated melatonin. This timing is affected by one’s chronotype.
16. It is the circadian rhythm that determines the ideal timing of a correctly structured and restorative sleep episode. Melatonin, the hormone from the pineal gland, called the “darkness hormone ” is of great importance in the functioning of the SCN. The most important target of melatonin in humans appears to be the SCN, as the SCN contains the highest density for melatonin receptors. A double effect of melatonin in the SCN, namely, an immediate effect and long-term effect, has encouraged its worldwide use against the ill effects of jet lag. This may not be wise to do.
As an immediate effect, melatonin is found to suppress neuronal SCN activity towards night time levels. During the daytime, the SCN neurons must run faster than normal. This is possible because the retina has more DHA in it than the brain. In terms of long-term effect, melatonin can phase shift and amplify circadian rhythmicity of the SCN. Melatonin application has been found to be useful in synchronizing the endogenous circadian rhythms not only in people who suffer from jet lag, but also in blind individuals, patients with dementia, and in shift workers. With seasonal changes in night duration, there are parallel changes in the duration of melatonin secretion, and this leads to more secretion in winter than as compared to summer. In the cold environments of fall and winter, melatonin couples to eNOS and not to light levels. In warm adapted humans in the tropics, the light remains the focus of SCN entrainment year round.
17. After the 4 hours of darkness, melatonin secretion increases and this allows plasma leptin to enter the hypothalamus if we are sensitive to its receptor. If we are leptin resistant, this process can no longer occur.
18. Once leptin enters and binds to its receptors, it affects the lateral hypothalamic tracts to immediately send a second messenger signal to the thyroid to signal it to up-regulate thyroid function and efficiency. This is how we can raise our basal metabolic rate when we are leptin sensitive. These coupled events, matched with leptin’s actions peripherally in muscles, occur at the UCP3 sites to burn fat as we sleep at a higher basal metabolic rate.
This means electron chain transport does not make ATP as usual. When leptin allows this uncoupling to occur, we make heat and not energy from normal metabolism. This means we will burn off our excess calories as pure heat. This is one reason why calories in and calories out argument makes no biologic sense once you understand how leptin works. Humans are built to burn fat at night as we sleep to lose excess weight we don’t need.
19. The timing of the leptin action is also critical. It usually occurs between 12-3 AM and is tied to when you last ate and how much darkness your retina (SCN) have seen. This generally occurs soon after our hypothalamus releases another hormone called prolactin from our pituitary gland in the brain.
20. The surge of Prolactin is normally quite large in normal darkness but is significantly diminished in artificially lit environments after sunset. This was shown in the Jessa Gamble video HERE.
This has big implications for modern humans. The reason is that prolactin release is coordinated with sleep cycles where autophagy is at its highest efficiency and where Growth Hormone is released. If this is diminished we generally see lower DHEA levels clinically and higher IL-6 levels on cytokine arrays. This is a measure of uncoupling of sleep from normal metabolism. I base every bio hack I do on this step in circadian biology because it is the most important.
21. The normal large circadian prolactin surge we should see at around midnight after leptin enters the brain, does not happen if the patient has leptin resistance, sleep apnea, or has eaten food too close (within 3-4 hours) to bedtime. This blocks leptins ability to enter the brain because of insulin spikes. As mentioned above, this step is usually impaired if you are a post-menopausal female as well. This is often why older women sleep badly and gain weight they can not seem to lose in the gym even with a good paleo template and good habits.
This is another reason I am a big advocate for bioidentical hormone optimization in women. This need is greatest in women who are warm adapted. The need is lowest in the cold-adapted females because their leptin levels are already low due to the cold. Postmenopausal women who are cold-adapted tend to do amazingly well clinically in most disease parameters in my clinical experience. The main problem they face is that their vanity and dogma keep them from using the cold pathways to become rockstars as they age.
Exercise training tends to frustrate postmenopausal women because if their hormone response is altered they have a lot of trouble as they age. Men, on the other hand, do not lose their GH levels until 50-55 years old usually. They are also protected by their testosterone levels which persist throughout life provided that they are not suffering from inflammation which directly lowers their free and total testosterone levels. GH and testosterone keep a mans heart and muscles in tip-top shape. If inflammation destroys these levels earlier in life, it can show up even in younger people. I am finding this clinical result is an epidemic in my own practice.
What happens when step 20 is broken in modern humans?
This commonly happens in diabetics, but it is now becoming a very common finding in modern humans because of the excessive use of technology after sunset. These artificial lights also tend to be quite bright and completely un-yoke the normal circadian signals from the hormone response. Light after sunset reduces the prolactin surge we normally see in humans. When we see chronic lowered prolactin surges we also see lower growth hormone secretion during the anabolic phases of sleep.
Lowered chronic GH secretion directly affects cardiac and skeletal muscle function because the process of autophagy is made less efficient as our life continues. Lowered GH and the sex steroid hormones at sleep lead to loss of cardiac function. This is why heart failure is strongly associated with low IGF-1 and sex steroid hormone levels. When growth hormone is not released in normal amounts, it also decreases our lean muscle mass and increases our fat percentage in all our organs and in our body. This leads to slowly declining organ dysfunction and poor body composition. We can measure this process clinically by looking for falling DHEA and GH/dopamine levels as we age.
What happens in normal aging in step 21?
Aging is among the most common features found in studies on modern humans when DHEA and GH craters on hormone panels. The loss of the prolactin surge is especially prominent in postmenopausal women. Most women begin to suffer from falling DHEA and GH levels around age 35-40 while they are still in peri-menopause. The higher their HS-CRP levels, the faster they enter peri-menopause and the quicker they enter menopause. They also age faster on a cellular level because their circadian chemical clocks are sped up. As a consequence, their telomeres shorten faster as well. Women have higher levels of leptin for childbearing, so they are more prone to leptin resistant issues than men. Leptin is a sexually dimorphic hormone.
This helps explain why older women struggle with cognitive haze, loss of body composition, poor sleep, and increased levels of heart disease after menopause. Many physicians think the losses they suffer are due to the loss of estrogen from ovarian failure, but the loss of growth hormone and progesterone production are far more significant in their physiology. Progesterone is the off switch to anything that is pro-growth. Modern women are usually estrogen dominant even after menopause because of mismatches in circadian biology. Cognitive loss is especially common in post-menopausal women. They also lose on average 1{a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6} of their bone mineral density per year from menopause in large part due to the loss of progesterone, not estrogen.
Loss of progesterone also corresponds to poor sleep in these women too. Replacing progesterone in women has a major effect on their sleep and bone stock. It also dramatically improves their memories and cognitive function as well.
Snacking after dinner… Effect on circadian cycles:
If you choose to eat within 4 hours of sleep you will never see the prolactin surge you need, because any spike in insulin turns off this critical sleep time release that corresponds to the cellular maximums of the autophagic process for humans. Agouti, the incretin gut hormone also rises in the blood to higher than normal levels to block leptin from entering the brain.
Diurnal cycles for agouti are coupled to NPY and have major effects on leptin. Agouti is a gene product that normally increases the release of leptin from fat cells at night to signal the brain of what the energy status is of the body. This is great when it is working well. When it is elevated due to heavy carbohydrate use in our diet it creates a massive problem. This is why late night carbohydrate snacking is a bad thing to do.
It appears 12-3 AM are the critical hours at night are where the remnants of mammalian hibernation lie for our species. These are the anabolic times for sleep when we are rebuilding our proteins and recycling our cellular contents. They are three of the most important hours in all human biology. If you miss them, you can bet you have several neolithic diseases for sure. Why do you ask? If these three hours are not reached enough during our sleep cycle, autophagy is never optimized and cellular repair does not occur in our cells. This means we are using old broken down parts in our cells as the next day arrives at 6 AM and cortisol rises again to wake us up.
We can measure this efficiency of this process by checking DHEA and IL-6 levels. I also like to measure hormone panels to see if the inflammation has destroyed any other hormone cascades in aging men or women. This is vital in taking care of older people and treating their longevity. IL-6 levels correspond to Leptin resistant states as well. This makes sleep and metabolic coupling tightly controlled by circadian biology at all times of our life. It is magnified because sleep gets worse as we age and our DHEA, HDL, and HS CRP rise. This is where, during a bio-hack, we can see why circadian mismatches can cause neolithic diseases in humans. Often times we can find the same issues develop much earlier in a young paleo person who has a lot of mismatches in their circadian biology. I test them the same way I would an older person.
Prolactin, Doc?
You must be asking, why is this prolactin hormone so important in a warm adapted human? Prolactin is not just a hormone that secretes human milk. That is the best-known action of prolactin, but not the most important. Immediately after prolactin is released during sleep, another signal is sent to the anterior pituitary to release the largest amount of Growth Hormone as we sleep (GH). GH is stimulated only during autophagic sleep cycles in stage 3 and 4 to increase protein synthesis for muscle growth while you’re dissipating heat via the uncoupling proteins. This is where the major release of GH occurs in humans post-puberty when they are warm adapted. 99.9{a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6} reading this blog are warm adapted. If you chose to become cold adapted the GH story radically changes, as laid out in CT-6. GH and dopamine are analog proteins.
The implications here are huge for the warm adapted human if this prolactin surge is not adequate to allow us to enter the anabolic stages of sleep. Prolactin surge is diminished by both artificial lights at night and by foods that stimulate NPY, (namely carbs and protein) when they are eaten in fall and winter when biology says they should not be available.

If you are leptin resistant for any reason, have sleep apnea, you will always have an altered body composition because of a low GH level and an altered sex steroid profiles on testing. The reason is that DHEA is the immediate precursor for those hormones and is always low in people with bad sleep efficiency. Most VLCers who are warm adapted face this very problem today. VLC diet is best used in the cold-adapted mammal and not the modern warm adapted lifestyle. In essence, this diet is a mismatch for our modern lifestyle. This is why so many bloggers think ketosis is a dirty word for performance and body composition.
This all implies that as you age you will have higher body fat {a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6}, lower muscle mass {a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6}, if autophagy is not optimized by great sleep. This is precisely what we see today in most modern humans as they age. Invariably, their sleep cycles and sleep durations are poor and decreased from their childhood levels. As they age, there is a chronic insidious erosion of circadian biology by decisions made by modern humans over and over again.
What about temperature variations in warm adapted humans?
Where does temperature enter the picture? In warm-blooded animals, homeotherms, such as humans, can change their metabolism in order to keep their heat production equal to the heat loss. Such animals have a temperature control system and thereby maintain a rather constant core temperature. Warm-blooded animals live with the advantage of an unchanged cell activity and temperature in their core. However, the human core temperature falls during the estrogen phase of the menstrual cycle (pro-growth) and during sleep (circadian rhythm by melatonin).
The lowest temperature of the day for modern humans is usually between 2 AM and 6 AM. The temperature cycle is part of the normal circadian periodicity. Our biological clock seems to be synchronized with the rotation of the globe daily. Meal composition and timing, light cycles and temperature play a role in altering normal cycles and autophagic optimization.
Ovulation releases a sharp rise in morning temperature with its estrogen surge. Progesterone effects seem to explain the higher temperature in the last phase of the menstrual cycle where it calms the pro growth effects of estrogen. In post-menopausal women, this balance is usually not ideal, and it leads to many menopausal complaints these women face today.
The reduced temperature induced by melatonin in sleep is needed for Central Nervous System autophagic repair, for another, less well-known reason. The lowered temperature sets the stage for the biologic quantum effects to be optimal on our neurons microtubules that facilitate learning and neuronal spouting that occurs brain-wide.
This is why if you don’t sleep well you feel bad the next morning and your mental performance suffers the next few days on cognitive tasks. Research also shows your learning is severely impaired because of lowered BDNF and changes in diurnal cortisol due to the sleep deficit. This is why we monitor truck drivers’ and airline pilots’ sleep and wake cycles by law!
Moreover, in hospitalized ICU patients or the elderly when this occurs, it sets the stage for the appearance of acute onset delirium. This is exacerbated when they also have a simultaneous cytokine storm from sepsis or obesity. We see this often in hospitalized patients who cannot sleep well in ICUs. Acute delirium states very much look the same as chronic sleep deprivation patients we see clinically as well. Inducing cold, using progesterone and using hypnotics helps manage these conditions. I mentioned this in my hour-long PaleoFX talk last week.
Okay, nonscientists take a breather. Geeks are up: So today we are going to look more closely at how circadian biology sculpts our species. We will assume the sun rises for us today at 6 AM. About two hours before the sunrise we are at our lowest body temperature and this signal is sent to our hypothalamus to the hypocretin neurons that link metabolism (leptin receptor) to the sleep cycle clocks. This temperature dip signals that sleep is coming to an end and that the brain needs to raise its cortisol levels to wake up the cerebral cortex not connected to the autonomic portions of the brain in the brainstem.
This is called the reticular activating system. When the reticular activating system is damaged, humans remain in a sleep-like state called coma. Neurosurgeons call this a chronic vegetative state. The release of cortisol is a neurochemical signal from the hypothalamus that allows the reticular activating system to wake up the cerebral cortex in the AM by increasing water flows from the CSF, Matrix, and cytosol.
Now we have to think about what season we are in? Is a long light cycle (summer) or is a short one that is cold (winter)?
VIP regulates the circadian rhythm in humans and most mammals. VIP is a gut hormone and is found in our taste receptors too! So if we taste the sweetness from carbs in our diet when it’s warm and they are growing in the environment, our brain is expecting us to be in a warm season rather than a cold one. So sweet means warm to the brain, not cold. If you mismatch that and eat carbs at the wrong seasonal time, you create inflammation in the brain and it throws off our chemical clocks in our cells and ages us faster. That means our telomeres get shorter. This is not good.
Even geekier: Taste perception and its relationship to glucose homeostasis begin with stimulation of taste cells located in tongue taste buds. There are five basic taste modalities: bitter, sweet, umami, salty, and sour. Taste cells are clustered into taste buds in the tongue epithelium. Mammals have four different types of taste cells (types I, II, III, and IV), exhibiting different molecular phenotypes and functional roles.
Type I cells are glial-like cells that maintain taste bud structure. Type II taste cells transduce sweet, bitter, or umami stimuli and communicate information through G-protein coupled transduction cascades. Type III cells synapse directly with afferent nerve fibers from three cranial nerves and most release serotonin upon depolarization. Type IV basal cells are rapidly dividing progenitor cells that differentiate into type I, II, and III cells. Along with biogenic amine neurotransmitters, it is becoming evident that multiple peptide hormones including glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK), and neuropeptide Y (NPY) as well as VIP are located in taste cells, potentially acting as signaling modulators of multiple gustatory stimuli.
The circadian clock not only can generate its own rhythms but can also be entrained by the environmental light-dark (LD) cycle. Multiple single-cell circadian oscillators that are present in the clock can, when synchronized, generate coordinated circadian outputs which ultimately regulate the overt rhythms.
VIP is a gut polypeptide, has been identified as one of the main neurotransmitters of SCN neurons, and participates in SCN function. These SCN neurons are retino-recipient and are found in the core of the SCN. They are activated by light, and exogenous application of VIP can reset the circadian clock in a manner similar to that of light application, both in vitro and in vivo. It is estimated that 9{a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6}-24 {a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6} of SCN neurons express VIP.
Leptin was originally described as an adipocyte-derived cytokine that signals to the hypothalamus to regulate food intake and energy expenditure. Leptin signals through its receptor, which is closely related to the gp130 cytokine receptor. Leptin can induce expression of the neuropeptide gene vasoactive intestinal peptide (VIP) through the VIP cytokine response element, the same element that mediates the response to the gp130 cytokines. Leptin acts synergistically with TGF-beta to activate transcription through this element.
One of the main chemical constituents of SCN neurons is the vasoactive intestinal polypeptide (VIP). Such neurons are retino-recipient and activated by light. Exogenous application of VIP resets the SCN circadian clock in a light-like manner both in vivo and in vitro. These resetting actions appear to be mediated through the VPAC2 receptor (a type of receptor for VIP). Unexpectedly, genetically ablating expression of the VPAC2 receptor renders the circadian clock arrhythmic at the molecular, neurophysiological and behavioral levels. These findings indicate that this intrinsic neuropeptide acting through the VPAC2 receptor participates in both resettings to light and maintenance of ongoing rhythmicity of the SCN.
Neurosurgery geeks only: In mammals, the part of the nervous system responsible for most circadian behavior can be localized to the suprachiasmatic nucleus (SCN). Although previous studies suggest that each SCN neuron may be an independent oscillator, these pacemaker cells must be synchronized to each other as well as to the environment to function adaptively. Therefore, answers to questions about cell-to-cell communication within the SCN lie at the core of understanding how his timing system operates. The daily cycle of light and dark is the dominant environmental cue responsible for synchronizing this biological timing system to the environment. The SCN neurons receive photic information directly from the retinal-hypothalamic tract (RHT). My Vermont 2017 video gets deep into the physics of the retina.
Many of the SCN neurons that receive retinal input from these cells are located in the ventrolateral (or core) region of the SCN and express GABA and, in many cases, vasoactive intestinal peptide (VIP) and the Peptide Histidine Isoleucine. These retino-recipient cells then convey this environmental information to the rest of the SCN. In brain slice preparations, application of VIP alters the firing rate of SCN neurons through a VPAC2 receptor-dependent mechanism and induces expression of mPer1 and mPer2 genes. These two genes are how the circadian cycles yoke directly to the cell cycle and are related to tumor suppressor genes and oncogenesis when mismatches occur chronically in modern man.
Functionally, the administration of VIP, and to a lesser extent PHI, can cause phase shifts of the circadian rhythms in vivo and in vitro in man.
The role of AVP (arginine/vasopressin) in circadian timekeeping has also been well established in the neurosurgery literature. Its role in the control of the circadian rhythm of food and water intake has been reported and well documented. Another intrinsic neuropeptide, VIP, acting through a VPAC2 receptor (a type of receptor for VIP), participates in both resetting to light and maintenance of ongoing rhythmicity of the SCN. NPY and GABA seem to be the neurotransmitters in the projection from the intergeniculate leaflet to the SCN adjacent to CN II. Raphe nuclei projections to the SCN contain serotonin as an NT. AVP and prokineticin 2 are seen in the outputs from the SCN as efferents.
NPY, which is an established neurotransmitter of the geniculohypothalamic tract (GHT), was found to regulate SCN neuronal activity and to produce long-lasting suppression of firing rate of SCN neurons. When co-applied with NPY, NT (neurotensin) was found to dampen the profound inhibitory effect of NPY. So when NPY is high, which would be in equatorial or high light conditions, NPY basically makes the SCN less efficient and allows animals to perform outside their normal circadian boundaries. They stay awake longer for eating and for reproduction in high light times during summer.
All geeks reunite: VIP (along with GRP and AVP) show circadian variations in the level of mRNA in constant contact with environmental conditions from our tongue and our gut. When light becomes long-lasting in summer, NPY dominates the SCN in mammals when light becomes low and the temperature falls to 50-55 degrees constantly at our surface cold receptors, and eNOS rises and blocks all photic input to SCN and circadian rhythms are maintained by a new program. Alpha MSH induces and potentiates that seasonal change within the hypothalamus as laid out in CT-6 blog.
The moral: So the brain is wired for foods when they grow naturally, not when we feel or think we can/should eat them regardless of their availability in modern times.
Leptin sensitivity directly regulates VIP production. VIP regulates the circadian rhythm and entrains the SCN to light. When it is cold, leptin is released from fat cells in large amounts, and we begin to use eNOS to entrain our SCN to cold cycles and we should avoid carbs like the plague then. Remember from CT-6, cold empties fat cells like screaming fire would empty a crowded cinema. In cold, the pituitary-hypothalamic portal is involved in the production of lots of alpha MSH and ACTH. When MSH rises, you are allowing the brain to control everything to get you to optimal. This should make it abundantly clear that cold and warm adapted mammals are not sharing the same circadian biology. Cold selects for supreme LS and superior hormone optimization as laid out in the CT 6 blog.
In long-light summer cycles, when VIP is controlling the SCN again, androgens normalize if the mammal is leptin sensitive. VIP usually fixes our Vitamin D level to optimal too. VIP is a master controller of all inflammation for circadian cycles, but leptin is the hormone that produces VIP in the correct amounts even in light cycles. So if we are leptin resistant for any reason in long-light cycles, we have no control over our circadian cycles and this leads to neolithic diseases.
Normally, VIP lowers our cytokines as the light cycle lessens as the day progresses. At night time the cell is more reduced and not as oxidized. Reduced means better cellular health and oxidized means more cellular inflammation. The act of cellular reduction happens in autophagy during sleep with repair processes. Remember VIP is highest in the morning and this helps it elevate cortisol to wake us up. This is also why cortisol levels are highest when we start our days and lowest in the night when we sleep.
VIP down-regulates most inflammatory cytokines and TGF-B1. It also raises activation of T-regulator cells of our immune system. All of the sickest patients have very low VIP levels and they also have changes in immune toll receptor proteins. Recently, researchers found that circadian rhythms influenced levels of an immune protein called Toll-like receptor 9, or TLR9, causing a daily peak and nadir. I have a sense these are affected by proton spin in cells. When mice were exposed to bacteria at different times of day, those who were infected at the low point of TLR9 activity developed severe sepsis and died much sooner than those exposed when TLR9 was high.
In another phase of the study, mice vaccinated near the daily TLR9 peak had stronger immune responses than those vaccinated at the circadian low point.
This implies those humans with sepsis, leptin resistance, and obesity all have poor circadian cycle control. It should be no mystery any longer why all of these groups suffer from collateral damage from other neolithic diseases. The risk of neolithic disease goes up in all those conditions. This is how mismatches can cause inflammation and shorten our telomeres in our cells. Remember circadian cycles are yoked to the cell cycle as I mentioned earlier in the CT series. This implies that any source of inflammation will shorten our telomeres and speed up our circadian clocks and result in neolithic disease and earlier death.
In the late night (4AM) when temperature levels are lowest, cortisol begins to rise in the blood while leptin levels in the blood are falling. Leptin levels peek in the blood at midnight to 2 AM. They are lowest in the morning when cortisol is rising. When leptin enters the brain and binds, this is the signal that causes the surge of prolactin that allows us to enter the two most important hours of human life when autophagy happens.
Leptin is a direct fatty acid synthase (FAS) inhibitor. Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis in humans. Its main function is to catalyze the synthesis of palmitate from acetyl-CoA and malonyl-CoA, in the presence of NADPH, into long-chain saturated fatty acids. Fatty Acid Synthase expression is stimulated by insulin, a hormone produced when blood glucose is high. Fatty Acid Synthase is inhibited by leptin, a hormone that has a role in regulating food intake and fat metabolism. Leptin is produced by fat cells in response to excess fat storage. Leptin regulates body weight by decreasing food intake, increasing energy expenditure, and inhibiting fatty acid synthesis.
Incretin gut hormones geek link: Leptin is an endogenous hormone released from fat cells and it is an appetite suppressant and stops hunger. Hunger is stimulated by the incretin gut hormone called ghrelin that is released from the stomach when light levels are rising and cortisol levels are rising. Recent evidence suggests that food deprivation, and the associated decrease in hypothalamic malonyl-CoA, increases the expression of neuropeptide Y (NPY) and agouti-related protein (AgRP), which stimulates ghrelin release in the stomach to produce the sensation of hunger. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Leptin activates the anorexigenic axis (appetite suppression) in the arcuate nucleus (ARC) of the hypothalamus by increasing the frequency of action potentials in the hypothalamic POMC neurons by depolarization through a nonspecific cation channel and by reduced inhibition by local orexigenic neuropeptide-Y (NPY) neurons.
The cold link: Why CT Simply Rocks
Cold temperatures sensitize us to leptin by causing it to be released from fat cells over time leading to a lower level in the blood chronically. Low temperatures also cause us to increase our RER while eating a low-calorie diet and still maintaining our lean skeletal muscle mass. Hydrogen motions in our matrix are the key to seasonal metabolic rate alternation patterns. These patterns are linked to the power density changes within seasonal EMF’s. The native EMF controls the second messenger calcium signals and free radicals inside of cells. Those signals control the flow of hydrogen isotopes in the matrix.
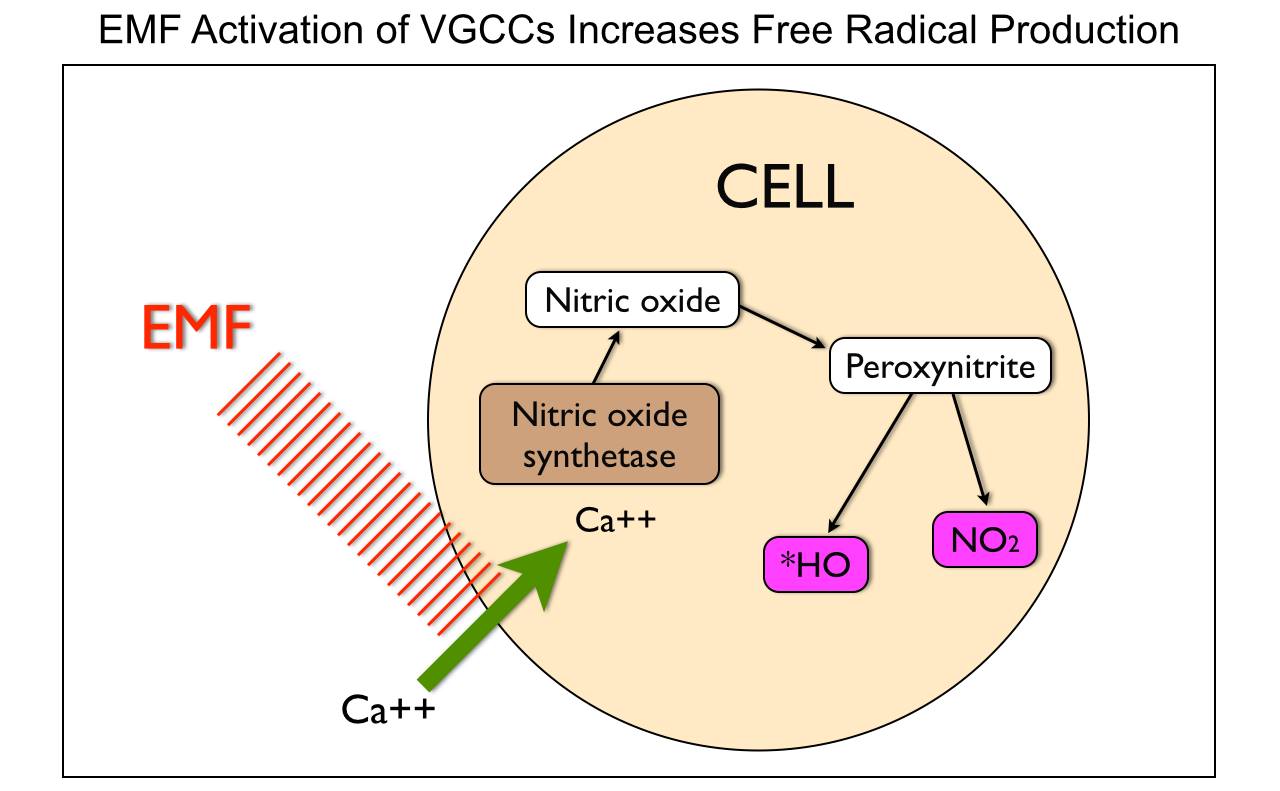
These findings show that during very-low-calorie diets, and low temperatures, a stimulant of a FAS inhibitor, like leptin, and would raise malonyl-CoA levels while decreasing the expression of NPY and AgRP. Clinically, this results in sustained satiation for longer periods of time with less food.
Remember that NPY is also the neuropeptide that is high in the SCN during high light levels when carbohydrates are highest. This peptide is directly regulated by leptin function. So if one is leptin resistant it appears to the SCN that winter has become summer. This is a circadian mismatch and a source of inflammation in the brain. When the cold comes and light drops eNOS is induced and shuts the SCN off to photic entrainment of the circadian clock. The reason for this is not only annual seasonality, but for periodic ice ages mammals have faced on earth and appear to be our primordial situation for life.
This is clearly a survival mechanism that is hardwired into all mammals by evolution, but the ancient pathway has another more important role that we have failed to uncover yet. (FACTOR X)
The environment required for the cold pathway expression is under cold, low light, and low-calorie conditions. All must all be met at once. This is precisely what all cold adapted eutherian mammals are ideally adapted to. These are modern human ancestors, and their biochemistry is foundational to our current paleolithic Ferrari engines. Many believe this pathway represents a starvation response (not), but its real biologic value is of even more interesting. We will talk about this later this year.
This temperature gradient gradually reduces all hunger, pain, thirst, and facilitate sleep in humans and all mammals. These functions were all selected for by evolution via natural selection pressures faced by eutherian mammalian evolution. Moreover, these effects of leptin cause specific epigenetic modification effects on the other hypothalamic hormones or peptides derived from POMC protein cleavage. Those changes are linked via the biology of the POMC neurons in the arcuate nucleus. Leptin and its receptor is especially sensitive to changes in temperature and to the light cycles that humans and all mammals face.
Leptin is intimately tied to hunger, it is linked to thyroid function and directly tied to fat metabolism in all mammals. Fatty acid synthesis, from acetyl-CoA and malonyl-CoA, occurs by a series of reactions that are in mammals catalyzed by individual domains of a very large polypeptide that includes an acyl carrier protein domain. Evolution of the mammalian Fatty Acid Synthase enzyme apparently has involved gene fusion in our evolutionary history. NADPH serves as an electron donor in the two reactions involving substrate reduction. The NADPH is produced mainly by the Pentose Phosphate Pathway. The H in NADPH needs to be a specific quantum spin state.
Every reunites for sleep and immunity: In the warm adapted human
Simultaneously, while sleep is rebuilding our cellular terroir (think levee one), the immune system is also undergoing autophagic repair as well. That is another reason why the temperature has to fall into our bodies. Usually, temperature rises and this causes the immune function to rise and more easily activate in response and duration in fever, stress, and infections. This activation depletes our immune system of its reserves during high light waking hours. Dropping our temperature as we sleep allows us to repair it. During sleep is when the body re-tools our immunity to function optimally the next day. What controls this entire orchestra of hormonal regulation? It’s all leptin-mediated and the brain is the master receptive organ to its function.
Sleep is a time for recycling and rebuilding to get us ready for the next day. It is also a time when our immune system is retooled to fight the battle the next day. Wound healing has been shown to be affected by sleep. A study conducted by Gumustekin et al. in 2004 shows sleep deprivation hinders the healing of burns on rats.
It has been shown that sleep deprivation affects the immune system function directly. In a study by Zager et al. in 2007, rats were deprived of sleep for 24 hours. When compared with a control group, the sleep-deprived rats’ blood tests indicated a 20{a7b724a0454d92c70890dedf5ec22a026af4df067c7b55aa6009b4d34d5da3c6} decrease in white blood cell count, a significant change in the immune system. It is now possible to state that “sleep loss impairs immune function and immune challenge alters sleep,” and it has been suggested that mammalian species which invest in longer sleep times are investing in the immune system, as species with the longer sleep times have higher white blood cell counts. Rats kept awake indefinitely develop skin lesions, hyperphagia, loss of body mass, hypothermia, and, eventually, fatal sepsis.
It has now been shown that sleep increases telomere lengths on leukocytes in humans. Sleep has also been theorized to effectively combat the accumulation of free radicals in the brain, by increasing the efficiency of endogenous antioxidant mechanisms. These mechanisms are mediated by the hormone DHEA which is the major antioxidant in the brain and correlates directly with effective sleep by lowering IL-6 levels. Progesterone is another critical hormone for brain homeostasis and learning as well. Sleep is vital to mammals, but it is supremely vital to humans because they have shrunk the benefits of hibernation into 2 short critical hours of their sleep cycle because of the massive growth of their brains extinguished the need to sleep through the winter months.
Since man can directly control his environment, therefore, being awake during winter was naturally selected for in his direct ancestors before the primates species because they have the same adaptations. The programs that control our fat mass (leptin) however still remain tied to our ability to sleep well.
Sleep geeks: The homeostatic sleep propensity (the need for sleep as a function of the amount of time elapsed since the last adequate sleep episode) must be balanced against the circadian element for satisfactory sleep. These things are controlled along with corresponding messages from the circadian clock; this tells the body it needs to sleep. Sleep duration is affected by the DEC2 gene. The optimal amount of sleep is not a meaningful concept unless the timing of that sleep is seen in relation to an individual’s circadian rhythms. A person’s major sleep episode is relatively inefficient and inadequate when it occurs at the “wrong” time of day; one should be asleep at least six hours before the lowest body temperature for optimal functioning. The timing is correct when the following two circadian markers occur after the middle of the sleep episode and before awakening:
- the maximum concentration of the hormone melatonin, and (you can test this but it’s hard)
- minimum core body temperature. (Easy to record with new Q self-apps on iPhones)
Sleep Implications:
A University of California, San Diego psychiatry study of more than one million adults found that people who live the longest self-report sleeping for six to seven hours each night. Another study of sleep duration and mortality risk in women showed similar results. Researchers at the University of Warwick and University College London have found that lack of sleep can more than double the risk of death from cardiovascular disease, but that too much sleep can also be associated with a doubling of the risk of death, though not primarily from cardiovascular disease. Professor Francesco Cappuccio said, “Short sleep has been shown to be a risk factor for weight gain, hypertension, and Type 2 diabetes, sometimes leading to mortality.
These all tie to a failure of autophagy in sleep stages 3 and 4 mentioned above. Here, we see why poor sleep links to sleep apnea and the neolithic diseases that are associated with sleep apnea. Growth Hormone is released in a pulsatile fashion from 12-3 AM during restorative sleep cycles 3 & 4, and this hormone facilitates autophagy and recycling of proteins. In essence, GH keeps us younger and in great shape when we sleep like a rock star. The problem is a modern man does not sleep well because of his brain’s technology and screen creations.
The metabolic phase during sleep at this time is anabolic which favors repair; anabolic hormones such as growth hormones (as mentioned above) are secreted preferentially during sleep. If things are working well. things get repaired at night as we sleep; and if sleep is poor, repair is either absent or suboptimal. When this occurs chronically, stem cells are used to replace cells instead of using cellular recycling processes that are normally used. Sleep is vital for all our organs rebuilding and retooling.
The human heart fails most commonly by autophagic failure. Heart disease is also the number one killer of both men and women so this means that physicians need to pay better attention to sleep. This is the most important question I assess in an initial bio hack. Autophagy, GH, and sex steroids all strengthen muscle fibers especially that of the cardiac muscle. Here we see the protective role of GH on the cardiac muscle once again.
Using the cold-adapted pathway described in CT 6, is the best way to protect from all circadian erosions, considering we no longer hibernate and have to rely on the two hours of anabolic sleep we get as a replacement. Cold lowers all inflammatory cytokines across the board.
In warm adapted humans, it becomes clear that inflammation is the single most destructive obstacle to human health. This implies that understanding how to control leptin becomes paramount for the warm adapted human.
Temperature variation in menstruating females:
The average temperature falls slightly from infancy to puberty and again from puberty to middle age, but after that stage is passed the temperature begins to rise again, and by about the eightieth year is as high as in infancy. In humans, a diurnal variation in temperature has been observed depending on the periods of rest and activity, lowest at 11 p.m. to 3 a.m. and peaking at 10 a.m. to 6 p.m.
During the follicular phase (which lasts from the first day of menstruation until the day of ovulation), the average basal body temperature in women ranges from 36.45 to 36.7 °C (97.6 to 98.1 °F). Within 24 hours of ovulation, women experience an elevation of 0.15 – 0.45 °C (0.2 – 0.9 °F) due to the increased metabolic rate caused by sharply elevated levels of progesterone. The temperature change is a sign of the movement of hydrogen isoforms moving between tissues in women. The basal body temperature ranges between 36.7 – 37.3°C (98.1 – 99.2°F) throughout the luteal phase and drops down to pre-ovulatory levels within a few days of menstruation. Women can chart this phenomenon to determine whether and when they are ovulating, so as to aid conception or contraception.
Temperature and light have massive biological effects on our biochemistry. We need to be aware of this.
More Support: Webinars by Dr. Kruse
- Factor X (May 2012)
Your Shopping List for this Post
Additional Resources
- Cold Thermogenesis Series
- Cold Thermogenesis 6: The Ancient Pathway
- My Leptin Prescription
- The Quilt: Autophagy
- The Paleo Summit: Is The Paleo Diet The Answer?






