The blog take home: Most people know that an elevated ferritin can lead to early aging and a lack of performance. Most people have no idea why. It is due to how iron and sunlight work or do not work together? Did you know that ferritin has a special protein coat that shield the iron crystals in the Fe (III) oxidation state? Did you know ferritin is PARAMAGNETIC? How might this be linked to its ability to cause disease? Any electromagnetic field causes molecules that are paramagnetic to be drawn towards that field. Could this be how ferritin is linked to many 21st century diseases? When iron cycles are not controlled in our blood plasma it can oxidize our mitochondria in tissues where this occurs. This lowers are redox power and can cause us to age faster. Here is a disease that gives you some insight how the science of Dr. Doug Wallace, Dr. Gerald Pollack’s ideas marry in human tissues. I will tie some of their ideas and experiments together in this blog, with a quantum view point, to help you understand how bio-physics works in cells to cause cataracts in this rare disease. This disease shows us that super high ferritin can be associated with a long life, but maybe correlate to diseases in tissues with high emissivity. This implies that ferritin may help people who lack solar exposure and magnetic flux from grounding because ferritin is a protein that moves charges in blood plasma.
Question I was asked by a member: Jack what you say to someone with Hyperferritin Cataract syndrome? The cause, best hacks, etc.?
Answer:
This is one of the rare human diseases that is 100% genetic and not epigenetic. I do beleive that the environment can speed up or slow down the genetic expression of this disease because of the experiments of Dr. Doug Wallace. As Wallace has said 80-85% of diseases are mitochondrial based in nature due to a lack of energy flows which turns on or off epigenetic expression. This disease is very similar to diseases like Cystic Fibrosis. They are both 100% genetic, but their phenotypic expression is made variable by the environment the person inhabits. Hyperferritinemia-cataract syndrome is caused by mutations to ferritin light chain (FTL) gene inherited as an autosomal dominant trait. Ferritin is a protein that binds and removes free iron from the blood plasma and tissues.
Mutations of the FTL gene in hyperferritinemia-cataract syndrome affect a region of the gene known as the iron regulatory element (IRE). A specialized protein called iron regulatory protein (IRP) normally binds to the IRE and suppresses the creation of ferritin. Mutations of the IRE prevent the binding of this protein and ultimately results in elevated levels of ferritin it the blood plasma. These patients can have astronical ferritin levels in their blood when a physician looks for it. Often this is how it is diagnosed because early on there are few other symptoms present until the cataracts show up later. Ferritin is also commonly found in the lenses of the eyes and if there is too much of it since ferritin has iron (Fe) in it with it massive arrays of D shell electrons it can absorb a lot of sunlight in the frequencies that are similar to hemoglobin. This is in the UV and IR range. Hemoglobin has a peak spectral absorption in the UV range at 280 mm and then at 420nm 540nm and 580nm and has sharp cutoff at 600 nm in the red range of the visible spectrum. This interaction leads to photoelectrically interactions with the light and these electrons can liberate more red light as heat. Heating does not uniquely equate to IR absorption as most assume. Light radiation entering hemoglobin and blood plasma drives charge movements in RBCs and water. This charge movements can generate secondary electromagnetic waves in these tissues which drive further charge movements. That heat is what causes the cataract excessive IR heat is a known to cause cataracts in iron workers in molten smelting. Most people think it is the heating from the smolting process that causes the cataracts but it is not. It may have more to do with medium that makes up the lens of the eye. The human lens itself lacks nerves, blood vessels, or connective tissue. So in this condition there is no ferritin in the lens but in tissues adjacent to it, there is a massive amount of ferritin and iron.
The largest elemental components of the body, by mass, are oxygen (65%), carbon (18%), hydrogen (10%), and nitrogen (3%). The other elements in the body, such as calcium, phosphorus, iron, and copper, are known to physiologists as mineral elements and trace elements.
The character of the medium is the key to understand in this process. The emissive character of the medium (lens) is expressed by researchers by a term called “spectral emissivity”. Objects with a higher emissivity radiate MORE energy than objects with lower emissivity. They seem more energized. This means the tissues around the lens re-emit much higher radiation and it is this secondary radiation in the IR range that cause opacification of the lens. If the tissues adjacent to the lens radiant emission happens to lie in the within the IR range (they do in humans), then objects with higher emissivity will appear brighter (whiter) in an IR camera than the surrounding tissues. You can see the areas around the eyes are whiter compared to the center of the eye where the lens is. The eyelid/orbicularis iris muscle has massive blood flows from the facial artery as you can see below. In this condition they would be loaded with ferritin and this would alter these tissues spectral emissivity relative to the the lens.

This is very important and I think a fatal flaw in modern cosmology when they speak about the expanding Universe discovered by Hubble’s red shift in his 1929 description. We can also see a red shift in thingds irrespective of their distance, if there are moving charges within the things we are imaging. This means that electrical plasma’s could easily simulate a red shift. The same effect is seen in cataracts, clouds, and in space in my opinion.
So the key hack is to limit your moving charges by maintaining a constant and chronic connection of Earth. This is one condition where specialize glasses blocking the spectral absorption of iron containing proteins may help. The problem with these glasses is they would also affect growth and metabolism pathways with blockade of UV and IR light. Ferritin is normally the storage protein that puts iron into the cells int he circulatory system under the direction of sunlight but the powerful sunlight in the cornea and lens is precisely what these patient need to avoid. Ferritin also is a highly charged human protein because of the iron and oxygen it contains. This is why cataract formation occurs in my opinion. This emissivity mechanism is why glutamate is so dangerous as well. It turns out the amino acid glutamic acid becomes highly charged when its side chains are altered to form glutamate. This is why glutamate is considered an excitatory neurotransmitter. Many people want to blame it for many diseases when it is the secondary re-emission of light from tissues that are the real cause of these diseases.
Because glutamate is highly charged protein, it draws secondary IR light emissions toward itself because of its moving charges. In this way, glutamate is like a light switch on cell membranes. It can turn on and turn off stimulation based upon the light another adjacent cell emits. If a cell emits too much light, like it does under duress, this causes too much excitation. It is not the glutamate that is the problem as some (Russel Blaylock) have postulated, instead, it is the excessive light released by stressed cells that is the key to the etiology of the pathology. This is why glutamate exerts its effects in mammals by binding to and activating cell surface receptors. Recall that all cells liberate ELF-UV light. Mitochondrial degradation of glutamate also contributes to insulin release when glutamate dehydrogenase is allosterically activated by solar light. High levels of glutamate and insulin can also cause cataract formation in humans. If you look at recent research papers on this topic glutamate itself is not the initial trigger for the exocytosis of insulin-containing granules. Most researchers have always assumed that the trigger is a glucose-evoked rise of ATP level closing ATP-gated K+ channels. The data don’t support this because the flux of glutamate through the secretory granules leads to the granules being more acidic and less positive in potential. They do not seem to understand that a low pH environment lowers the EZ of cell water. It appears that the low pH and lack of EZ is what really modulates the amount of insulin release occurring from cells. The EZ is dynamic and not static, in it’s size and charge. It responds by increasing or decreasing its size and charge as the things that build it or destroy it. This is why I beleive diabetes really is a a disease of a lack of solar exposure. Researchers have made initial claims that glutamate potentiated secretion were based on the observation that a rise in cytoplasmic glutamate concentration correlated with increased insulin release, but were disputed in several papers, when it was found that insulin release did not always correlate with the total islet glutamate concentration. Unfortunately none of these papers controlled for light emissions or the light spectrums in those labs. If they had, they would have seen why insulin is a solar hormone and glutamate is an optical light switch for this mechanism. These discrepancies have suggested that it is not so much the total islet, nor even cytoplasmic glutamate concentration that is the important regulatory factor, but the flux of glutamate through the secretory granules, which regulates the granule pH and membrane potential. Here again you see pH and voltage are functions of the size of the exclusion zone of water locally in a cell. My sense is that man made artifical light increases glutamate and insulin action specifically because blue light frequencies dramatic lower voltages on the inner mitochondrial membrane, change the 100 Hz vibration a mitochondria needs to fat burn, while also slowing electron chain tunneling speeds resulting in low NAD+ at cytochrome 1 and causing a pseudohypoxia that favors a Warburg like metabolism.
Why is all this important? It links to how photosynthesis works in quantum fashion. The first step in photosynthesis and in the blood plasma where chlorphyll and hemoglobin are is the charge separation of water to make electrons and liberate hydrogen. Hydrogen is a clean natural fuel. Have a look at the chorophyll and hemoglobin molecule picture that was posted in Time #21. Note the similarity of the nitrogen cage and carbon skeleton. Here you can see the doped semiconductor base with a different metal ion at is core. Iron bonds oxygen well and magnesium does not. This is why human RBC’s bind oxygen well that plants exhale. This “photosynthetic quirk” in one atom at the center of the porphyrin cage is what couples plants and animals perfectly. This is why plants evolved before animals. Animals could not have come first because there was no oxygen to drive their mitochondria to power their more complex systems. It is a precise quantized reaction among these living cells.
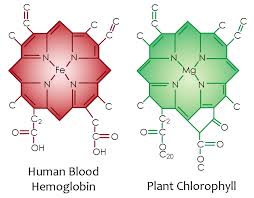
Plants use photosynthesis to charge separate water and to turn water, carbon dioxide and sunlight into oxygen and the energy they need to power their systems. The first step in photosynthesis and blood plasma is charge separartion of water. In a leaf and in blood water will absorb and emit all radiations from chlorophyll and hemoglobin to build a massive EZ in water in a leaf and the blood plasma. A large EZ drives flow through hydrophilic tubes in leafs and in the circulatory system. Pollack has proven this flow exists in his experiments with hydrophilic tubes in water. The iron in hemoglobin provides more electrons to make a larger EZ in the circulatory system than we see in a leaf because RBC’s are larger than the capillaries through which they pass. The energy of the sun is also used to contort these cells as they pass so that there is a zero resistance to flow. In leaves and blood the EZ functions to store potential energy for subsequent physiologic work. Iron allows for more more work to be done because it has more electrons for sunlight to collide with photoelectrically. And for decades scientists have been trying to replicate this reaction in order to create biological systems that can produce cheap, clean hydrogen fuel. Hydrogen is the ultimate atomic energy chameleon for all living things.
Hydrogen has the potential to be an energy efficient replacement for many of the things that we currently rely on. If you listened to the April 2016 webinar you heard me talk for three hours about how the mitochondrial matrix collects H+ ions in close proximity, at a small scale, to do some amazing things. One such thing is the creation of a transient magnetic monopole. This has the power to create an unlimited energy source to power life when energy is required. They key is to keep charges moving while the scale of action is shrunk at the same time in thin sections adjacent to water. Plants create hydrogen in their leafs via photosynthesis and use this hydrogen as a clean fuel. But up until recently, man hasn’t been able to find a way to create it as safely and efficiently as plants do. I have always believed that eukaryotes, like humans, have the ability to use a modified version of photosynthesis on top of our skin, within our skin (D3), in our RBC’s and inside our circulatory system close to our surface. We use all iron containing proteins to do this. To replicate this step in the reaction in plants, research teams have taken ferritin, and modified its chemical and electrical structures slightly. I have a sense this is why this disease was created by nature.

More ferritin production inside vessles would be an adaptation to a low quantum yield environment. It is a remnant back to when humans were trying to maximize energy production from the sun. This bottleneck seems to have occured 70,000 -100,000 years ago. Unfortunately for this disease process, when the sun returned to full power, the highly charged ferritin in a strong solar environment became toxic for the eyes and leads to cataract formation. The interesting thing is that the development of the congential cataracts is the only real problem these patients get. This tells me that it is an epigenetic disease related to a very chronic low quantum yield environment that humans may have faced in the past. It also maybe an adaptation for life at high latitudes. Other symptoms of the disease also seems to link to disease linked to a low quantum yield. Those conditions are related to hypothyroidism, fatigue and arthritis but most of these patients can live a relatively life if the cataract is treated once it forms.

Discussion:
Energetics in animals is based on the availability of reducing equivalents, specifically hydrogen (H+) which is replenished byconsumed as carbohydrates and fats, that react with oxygen to generate water and hydrogen via mitochondrial oxidative phosphorylation.
The older evolutionary pathways makes far less energy than complex animals need for chronic living. Glucose is cleaved into pyruvate via glycolysis within the cytosol, reducing cytosolic NAD+ to NADH (reduced nicotinamide adenine nucleotide). The pyruvate then enters the mitochondrion via pyruvate dehydrogenase (PDH), resulting in mitochondrial acetyl-CoA (main electron provider for ECT), NADH + H+, and CO2. The acetyl-CoA then enters the tricarboxylic acid (TCA) cycle, which strips the hydrogens from the organic acids, thereby generating NADH + H+.
The new pathways (beta oxidation) are required for complex life because they provide a lot more electrons for tunneling. The more electrons the more life you live.
Fatty acids are oxidized entirely within the mitochondrion by beta oxidation, generating mitochondrial acetyl-CoA, NADH + H+, and FADH2; the latter is contained in the electron transfer factor. Two electrons are transferred from NADH + H+ to NADH dehydrogenase (complex I) or from FADH2-containing enzymes such as the electron transfer factor dehydrogenase or succinate dehydrogenase (complex II) to reduce ubiquinone [coenzyme Q10 (CoQ)] to ubisemiquinone, and then to ubiquinol. This is where the Q-cycle becomes important. This is why the Time #21 blog explodes the biophysics here at this step. The electrons from ubiquinol are then transferred down the remainder of the electron transport chain (ETC) successively to complex III (bc1 complex), cytochrome c, complex IV [cytochrome c oxidase (COX)], and finally to oxygen to yield H2O. The water is critical in forming the main battery for light………the EZ. Cytochrome c oxidase is a heme containing protein.
This is the photosynthetic step that Wallace misses because he does not seem to know about Pollack’s work. Wallace still believes in chemiosmosis as the key energic step in a cell and I no longer do because of Ling and Pollack. Ling’s calculation that we need 5000 times more ATP than a cell can provide make it difficult to accept Mitchell’s theory that chemiosmosis explains a cell’s energy needs. In my view, they are no longer tenable given what Gerald Pollack has found in the EZ experiments on water. While, chemiosmosis is still an important step in cells (unfolding or proteins to bind water made in mitochondria)……the formation of water for the cytosol is much more critical for energy production. Not only that, EZ water can store light for years. Piccardi and Voeikov work discussed on pg. 107 Pollack’s water book is the missing data in Wallace’s bio-energetic paradigm.
What Wallace still believes: The energy that is released as the electrons flow down the ETC is used to pump protons out across the mitochondrial inner membrane through complexes I, III, and IV. This creates a proton electrochemical gradient (ΔP = ΔΨ + ΔµH+), a capacitor that is acidic and positive in the intermembrane space and negative and alkaline on the matrix side. The matrix side is where the TCA cycle occurs. The alkaline pH here increases the EZ as Pollack has proven. The matrix is also very gel/colloid like which also completely fits Pollack’s experiments. Realize Pollack’s work calls the EZ a capacitor for UV/IR light that stores the potential energy of light for very long times. The EZ is DESTROYED by acid pH and protons are excluded in the intermembrane space of the mitochondria. This is radically different than what Wallace currently believes and is why Wallace is so close yet so far. Wallace believes that the potential energy stored in ΔProton gradient is used for multiple purposes: (a) to import proteins and Ca2+ into the mitochondrion (I believe to regulate the 100 Hz requirement for beta oxidation (b) to generate heat, (to make the EZ and act as a heat sink for the Q -cycle) and (c) to synthesize ATP within the mitochondrial matrix (my view point is this is the doping mechanism for carbon based semiconduction). The energy to convert ADP + Pi to ATP; The ATP, in my view, is used to unfold proteins to allow water created by mitochondria to bond to it to make a large EZ from the heat liberated from the mitochondria. The ATP comes from the flow of protons through the ATP synthetase (complex V) back into the gel/colloid matrix. I think red light around the ATPase may allow for proton tunneling without need for transport back thru the ATPase at night using light emitted from our own semiconductors. Light tends to draw particles together when the light is intense and powerful and a laser fits that bill. The gel/colloid mitochondrial matrix ATP is then exchanged for cytosolic ADP by the inner-membrane adenine nucleotide translocators (ANT’s).
The efficiency with which dietary reducing equivalents are converted to ATP by OXPHOS is known as the coupling efficiency. I believe this is linked to mitochondrial haplotypes we inherit from our maternal line based upon location linear and the photosynthetic quantum yield of this location. SNP’s are post translation modification of the mitochondrial haplotype that allowed for migration from the East African rift. Wallace has not said this, as far as I can tell. Wallace believes that this is determined by the efficiency with which protons are pumped out of the matrix by complexes I, III, and IV and by the efficiency with which proton flux through complex V is converted to ATP. This brings uncoupling proteins into the equation. I also believe uncoupling proteins and SNP’s are what allowed for migrations. The uncoupler drug 2,4-dinitrophenol and the nDNA-encoded uncoupler proteins 1, 2, and 3 render the mitochondrial inner membrane “leaky” for protons. This lowers the voltage of the inner mitochondrial membrane and it should short out the Mitchell/Wallace’s version of a capacitor. I believe if he knew about how solar light forms a capacitor in water to make an EZ, I wonder how he would alter his view his current conception of bio-energics. His verison however still gets the overall story correct that energy deficits lead directly to disease propagation without any input from the nuclear genome. All we need to see for a disease to manifest is a change in energy flux from a mitochondria. This is the critical piece of what causes a disease phenotype to emerge from no where.
When the proton gradient shorts out the system this short-circuits the mitochondrial inner-membrane capacitor; uncouples electron transport from ATP synthesis; and causes the ETC to run at its maximum rate (high voltage), thereby dissipating the energy as heat. My idea is that the high voltages created by uncoupling would lower semiconduction current in the Q cycle of the mitochondria and create a spectral shift in the light that excited the electrons to begin with. This would liberate massive amounts of heat for a mammal and this is where warm bloodednessevolved. The release of large amounts of red light/heat would immediately build a large EZ. Red light also stimulated the 4th cytochrome directly because it is a heme protein that responds to “some of the same frequencies” that hemoglobin does. The same thing is true for the ATPase. This means red light emission during uncoupling could still make ATP without a need for more electrons from foods. This would optically augment magnetic flux because the ATPase would become a perfect quantum rotatory engine. This has been proven already. The key is that semiconductive light emission would cease in the Q-cycle and this would affect sleep and memory function. This is how many neurodegenerative and neolithic diseases begin. It is also how eye diseases would manifest that lead to other diseases as the central retinal pathways are slowly destroyed.
Variation in mitochondrial proteins (mt haplotype) has been proposed to alter the OXPHOS coupling efficiency, thus altering the proportion of the calories burned by the mitochondrion that are allocated to ATP generation versus heat production. I think it is way more complicated than that. I think SNP’s and the ability to emit light from the Q-cycle are the most critical broken steps in mitochondria that lower our ability to make energy at low cost by generation of a transient magnetic monopole who’s flux would be stored in the EZ of the cytosol to drive bio-energenics when coupling efficiency fails for many different reasons. Loss of beta oxidation from the environment obviously topping the list. Alterations in the coupling efficiency can influence, bio-photon release, ROS generation, modulating Ca2+ uptake, and predilection to apoptosis. This is not good in the brain or heart but is well tolerated in the liver. In the liver when the process is chronic this causes diabetes. In the heart we see heart failure and coronary heart disease (CAD). In the brain we see a slew of neurodegenerative diseases as the tissue mitochondria lose their ability to make energy. We also can see why now cardiac function and brain function are linked via their mitochrondria capacity. A cataract formation in these patient results from the loss of energy production in the anterior chamber of the eye because the surrounding tissues absorb and re-emit massive amounts of IR light that causes lens opacity. Such physiological changes permitted our ancestors to adapt to a range of new poorly lit solar environments when they all acted in unison; today they are all disrupted by blue light and massive variable electromagnetic fields that act upon these mitochondrial memchanisms.
When you understand the above fully you’ll see why I am no fan of exogenous supplements of anti-oxidants. Why? A “mitochondriac” understands that the system is non linear and far from equillibrium. It uses part of light’s non linear physical attributes to fine tune cycles that are coupled into positive and negative feedback loops that require little energy to run. Time 21 gave you one in analogy by using Rayleigh-Benard cycling. This type of cycling is critically used in the eye because when it is broken the surface cornea, lids, and orbicularis iris muscles heat up and re-emit light to the adjacent lens causing opacification.
Given that mitochondrial dysfunction involves a perturbation of metabolism, why have metabolic and pharmacological treatments not been more effective? We believe that the answer lies in the integrated network of interactions within the human cell, in which mitochondrial bioenergetics is involved. A single energetic defect can have many different consequences. Most of these consequences a food guru is blind to becaus their focus is myopic with respect to light. Thus, use of a single metabolite such as ascorbate (vitamin C) or tocopherol (vitamin E) to perturb the system may have only a transient effect, given that the other components of the network ultimately counteract the therapeutic intervention. Furthermore, the varying energetic defects in different patients perturb the network in different ways, because they are NON LINEAR by nature. Hence, the same therapeutic approach may be beneficial for one individual and deleterious for another. This is why bio-physics is a N =1 game.
To understand mitochondrial bioenergetics, we must start with the source of our energy: the dietary calories that we consume. Those electrons are programmed by the sun. The environmental calories consumed ( electrons programmed by the sun via the photosynthetic web) determine the availability of reducing equivalents for the mitochondrial redox reactions.
Wallace’s view is interesting: “In the presence of excess calories/electrons, cells are programmed to actively catabolize the available calories and use the energy for cellular growth, maintenance, repair, and—in the case of proliferative cells—to replicate their DNA and divide.” He hypothesized that the energetic status of the cell is communicated to the nucleus (my beliefs this is done wirelessly) by the modification of nuclear chromatin through phosphorylation via ATP, through acetylation via acetyl-CoA, and through methylation via S-adenosylmethionine—all high-energy intermediates generated via glycolysis and mitochondrial metabolic and OXPHOS pathways. Why do I think it is wireless communication? What do chromatin histones and methyaltion fundamentally all do in cells from a physical perspective? It coils DNA and RNA. Why is that important? Buried deep in Time 21 lies the answer. When any coil is coupled to capacitor that resonantes at a specific frequency wireless tranmission is possible. Who proved that this was the basic tools needed for wireless communication? Tesla did when he built his first Tesla coil. Tesla used induction coils and capacitors, a device known as a Leyden jar to store static electricity to give split phase AC currents to run his induction motors. In cells, mitochondria have replaced Leyden jars to store a 30 million volt charge.

Mitochondria do exactly the same thing but they generate DC electric currents because they use water as their main capacitor. This is why Wallace needs Pollack’s perspective and should not rely too much on Peter Mitchell’s perspective that the proton gradient is the main capacitor in mitochondria. Water is what does this in nature with the addition of any light source.
This is what makes me different from both of these men. I see what both see, but I observe how they are connected by nature in quantized fashion to do and explain what Becker found in his critical experiments in the 1960’s. This is the theoretical basis of all the science buried in Time #21. That should give you a lot to chew on today.
CITES:
- http://www.bloodjournal.org/content/90/2/814?sso-checked=true
- http://www.sciencealert.com/australian-scientists-have-replicated-a-key-reaction-that-allows-plants-to-convert-sunlight-and-water-into-fuel
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407.
- Wallace DC. Mitochondria as chi. Genetics. 2008;179:727–735.
- Wallace DC, Lott MT, Procaccio V. Mitochondrial genes in degenerative diseases, cancer and aging. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin’s Principles and Practice of Medical Genetics. 5th ed. Vol. 1. Philadelphia: Churchill Livingstone Elsevier; 2007. pp. 194–298.
- Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719.
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430.
- Wallace DC, Zheng X, Lott MT, Shoffner JM, Hodge JA, et al. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988;55:601–610.
- Maechler P, Wollheim CB (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402: 685–689.
- Hoy M, Maechler P, Efanov AM, Wollheim CB, Berggren PO, et al. (2002) Increase in cellular glutamate levels stimulates exocytosis in pancreatic beta-cells. FEBS Lett 531: 199–203.
- Rubi B, Ishihara H, Hegardt FG, Wollheim CB, Maechler P (2001) GAD65-mediated glutamate decarboxylation reduces glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem 276: 36391–36396.
- MacDonald MJ, Fahien LA (2000) Glutamate is not a messenger in insulin secretion. J Biol Chem 275: 34025–34027.
- Bertrand G, Ishiyama N, Nenquin M, Ravier MA, Henquin JC (2002) The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J Biol Chem 277: 32883–32891.
- Casimir M, Rubi B, Frigerio F, Chaffard G, Maechler PSilencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J 2009 Dec 10;424(3): 459–66.


Dr Jack Kruse Thank you!!!!