READERS SUMMARY
1. WHAT IS A PRION AND WHY IS IT IMPORTANT?
2. BEWARE OF GARY TAUBES ?
3. HOW DOES SHAPE, SIZE, LENGTH OF PROTEIN SCALE TO BIOLOGIC ENERGY?
4. HOW DOES THE PHOTOELECTRIC EFFECT WORK IN US?
5. WHAT IS THE FOURTH PHASE OF MATTER, AND WHY DOES IT MATTER TO US?
Prion diseases, also known as spongiform encephalopathies, are fatal neurodegenerative diseases that can be sporadic, genetically determined, or acquired by a so called “infection”. One of the hallmarks of prion diseases is the cerebral accumulation of an abnormal form of prion protein (PrP), denoted PrPsc, which is derived from the normal cell surface glycoprotein (PrPc).
Many of you might be unaware that the 1997 Noble Prize was given for this discovery. Even fewer of you know that one of the “ancestral leaders” now running NuSi tried to obfuscate this scientist’s reputation in a 1986 article in Discover Magazine. This is where I first heard of Gary Taubes. It’s also why I became wary of his ideas and his writing methods and his goals. He followed his first defamatory article in 1986 with another in 1997 by writing another highly inflammatory account of this discovery. This was after Dr. Prusiner received his Noble Prize in 1997. It is time you become aware of Mr. Taubes’ history. When science writers do not agree with a scientist, they allow their own personal biases to dictate their reports. You need to exercise due diligence in regard to what they write. Ever since these two articles were written, I have been a skeptic of Taubes’ position on many things. His agenda tends to set his words in the printing press. This is why I have remained ‘luke warm’ on NuSi goals, but I have kept an open mind unlike Taubes did about prions.
Many of you know that I have also called out 5 other Nobel Laureates for supporting a false paradigm. Today’s blog is about a Noble Laureate getting a prize in medicine that he deserved. The reason he was mocked is because he did not provide a mechanism for his discovery. He is the only person I have found who tripped over the “quantum mechanism” by accident. Even to this day he remains in the dark about how it actually works.
THE PRION QUANTUM MECHANISM
Today, the mechanism for the conformational prion conversion is speculated to be an elusive ligand-protein, but no such compound has been identified to this point. A large body of research has developed on candidates and their interaction with the PrPC. Presently, copper is the only confirmed ligand involved in this process, though the implications of this knowledge are a matter of much debate; the NH2-tail region has been shown to bind Cu2+. The binding caused a conformational change by some “unknown effect”. This blog will show you how that effect manifests. The copper-PrP interaction has been linked to resistance of oxidative stress. If you read OSF 3, none of this speculation should surprise you. PrP appears to be a copper buffer for proper neuron and synaptic cleft function. I will remind you that copper is a transition metal that has a very special D electron shell. In this role, the normal prion protein could serve as either a copper homeostasis mechanism, a calcium modulator, or a sensor for copper or oxidative stress. PrP is present in both pre- and post-synaptic neuron cells, and the greatest concentration is in the pre-synaptic cells. It is also found in the synapse. PrP seems to be able to shuttle ionic copper from outside the cell to the interior of the cell for physiologic use. The ability to bind copper is pH-dependent, and linking its function with water micelles around the PrP protein is critically important. However, a critical limitation of all these studies is that they have been performed in an aqueous solution, whereas PrPC in a living cell is associated with cellular membranes and microtubules in an aqueous environment. This is why the mechanism has eluded science, in my humble opinion. NMR data normally shows copper binding results in a conformational change at the N-terminus of the PrP protein.
PHYSICS GEEKS: Robert O. Becker associated the mammalian life system with DC currents in his work to show that all mammals use carbon based semiconduction to generate energy for uses in bone, regeneration, and in wakefulness. Today’s blog raises this question: could the effective semiconductor property in the brain be due to the fact that the transfer of charge carriers from DHA to water occurs in a smaller space-time sheet? Remember, water is naturally confined by microtubules in the brain. Might the brain act by first accelerating electrons in the pi electron electric fields in DHA? This becomes analogous to the transfer of electrons between conduction bands in a semiconductor junction called the Josephson junction. If the answer to my question is yes, the semiconductor property of DHA, iodine, and water would be a direct signature of the realization of how metabolic energy quanta is transformed to a zero point kinetic energy as water moves down a microtubule. PrP is a critical protein in the transfer of energies in this process. For metabolic energy quantum of order .5 eV, it would only make sense if the electrons can be transferred to the smaller space-time sheet. This would require energy slightly below the minimum energy for the transfer to the smaller space-time sheet in the absence of the Becker potential. Microtubules allow for this process in the brain. All neurons are loaded with microtubules. The construction of the neuron would be critical in this situation. Moreover, the 1 mV voltage generated by the DHA electric field could serve as a “control knob” for this process. Interestingly enough, all neurodegenerative disorders are associated with poor voltage on EEGs. So this begins to make sense.
When voltages drop in the brain, cerebral blood flow slows dramatically and BP rises. This has major thermodynamic affects. Remember, blood cells are filled with hemoglobin. Hemoglobin is loaded with another transition metal called iron. Hemoglobin acts like a chromophobe in the brain as well. I told you this in OSF 3. Why is this important? Because it brings optics into the equation of how we generate energy in the brain’s microtubules. Iron content affects water chemistry in a huge way. When iron levels drop in the brain, blood flow decreases and we lose oxygen tensions. The amount of protons rise in CSF which increases the temperature and the mass of our CSF. Pollack showed us that warm water has a higher mass than cold water because it has more protons. Consider this analogy to our oceans: When sea water temperature rises for any environmental reason, oxygen diffuses out of it. When your temperature rises, oxygen diffuses out of your tissues, too! These are natural chemical and physical laws of the universe, people. Biology is not immune to these laws. Biologists just act as if it is. When oxygen drops in the ocean, so do iron levels in sea water. When iron falls in you, so does your tissue oxygenation. This is why blood pressure rises in all neurodegenerative disorders. The brain is trying to get iron back for some reason. Why? It is to improve the optics of CSF and the water inside neurons using the magnetic effects of iron to form magnetic flux tubes in microtubules.
Do you know what happens to sea water when iron falls? It changes the color of the water. This is why hemoglobin and bilirubin are both chromophobes in all tissues. A loss of iron can paradoxically make the ocean water a crystal clear blue, but this will make it devoid of life. Remember in most mental illness, neuro-degeneration, and autoimmunity iron and copper metabolism is altered in many different tissues. Anyone who has seen the water around the tropics or the equator knows the water there has always been extremely blue. It is not blue the further you get away from the equator. What else should you know about blue sea water? The more blue sea water is, the less life it contains. The further north or south you go from the equator, the greener the ocean water. When iron is present in abundance, algae can thrive. Algae makes DHA from water, sunlight, and proteins and lipids in the sea. DHA feeds the base of the marine food chain everywhere on this planet. Green water is filled with “life” that creates DHA. DHA is life’s ultimate an electron collector.
BRAIN CURRENTS
In the brain, there are pairs of structures adjacent to one another in a positive and negative potential at various scales based upon the currents that flow within them. It turns out that the current between these alternatively charged plates form an effective capacitor. This capacitor can flow when the current is above some minimal potential difference between the plates. In the brain, the plates are the cell membranes and the water inside the neurons. This water flows in the microtubules. PrP is bound to the cell membrane in all neurons, and in the synaptic cleft, to maintain this electron current. The current flows from a positive to a negative pole based upon Becker’s data. This is an electron current. One must also consider the possibility that a proton current might flow in the opposite direction to direct this voltage. This is because water naturally charge separates to form a large layer of hydronium ions (protons) when it is adjacent to hydrophilic proteins within a neuron. Becker’s work considered both, but he found that the DC electron current was experimentally favored. His most shocking proof came when he used a magnet to stop electron flow on the neocortex in animals. He experimentally showed that consciousness is lost when a magnetic field is used to deflect the electric current in the brain. We can see why PrP is critical to electron currents in the brain. It controls the electrons donated by copper to the DHA lipid membrane. This, in turn, maintains the electrical gradients in the brain. This also implies that PrP would be intimately tied to circadian regulation in the brain. Why?
NON GEEKS: Prp is critical to circadian regulation in the brain. PrP mRNA has been demonstrated to cycle regularly with day-night. Given the diversity of interactions, effects, and distribution, PrP has been proposed as a dynamic surface protein that functions in many neural signaling pathways. Specific sites along the protein bind other proteins, biomolecules, and metals. These interfaces allow specific sets of cells to communicate based on level of expression and the surrounding microenvironment. PrP acts in concert with many anchoring proteins on a GPI lipid raft in the brain’s DHA lipid bilayers of neurons. This all supports the claims that PrP is a critical protein in providing an extracellular scaffolding function. This protein builds and directs neurogenesis in the human nervous system by controlling the electron current on the cell membrane.
In the Energy and Epigenetic series, I showed you that neurogenesis and the immune system are linked via the MHC 1 gene. Though most attention is focused on PrP’s presence in the nervous system, few seem to know it is also abundant in immune system tissue. Here you can see how the MHC 1 gene links the brain and immune systems together from an evolutionary perspective. PrP immune cells include hematopoietic stem cells, mature lymphoid and myeloid compartments, and certain lymphocytes; it has also been detected in natural killer cells, platelets, and monocytes. T cell activation is accompanied by a strong up-regulation of PrP, though it is not requisite.
WHERE PRIONS ENTER THIS DANCE
The lack of immuno-response to transmissible spongiform encephalopathy (TSE), also known as neurodegenerative diseases caused by prions, is directly linked to the protein conformational change to PrPsc by alterations in quanta of energy delivered to certain domains of the prion. When the prion is in the Prpsc form, it cannot up-regulate immune function as the normal PrP does.
In OSF 3, you heard about how I postulated that DHA has dictated 600 million years of mammalian evolution without relying on DNA or RNA. Today you are going to learn about how chaperone prion proteins (PrPc) that have been liberated from the native electromagnetic force can be transformed by higher energized electromagnetic forces, creating emergent neurodegenerative diseases and autoimmune diseases. In other words, PrPc becomes PrPsc to cause diseases.
They key is that the amount of quanta added to the protein determines its physiologic ability. Size, shape, and energy determine what a protein is capable of. The addition and subtraction of energy quanta is what determines shape and function based upon mass equivalence. When electrons are lost from cysteine disulfide bonds, it directly alters the conversion of alpha helices to beta amyloid sheets that cause prion diseases. Evidence for the multiple conformation states of proteins is now abundant in the literature. Back in the 1980s and 1990s it was unheard of. What is still not well understood in today’s world is how this affects protein function and how it influences tissue levels changing at a quantum level. It is not the DNA code, as most believe today, that performs this task.
All of you should already know the basics of how the quantum mechanism operates. It is documented in OSF 3 in detail. Today, I am going to show you how PrP was behind the process that drove evolution of the human neocortex without much genetic alteration of chimp DNA or RNA. The natural process that drove us from ape to human was using chaperone proteins, like PrP, under the native electromagnetic force. This created small emergent proteins that interacted together with water chemistry in our microtubules to alter our evolutionary trajectory. Prion diseases are the result of a breakdown of that very fundamental process. PrP directly controls electrical currents in the neocortex to act as a SQUID. This directs electrons vertically into neural circuits in the brain from the horizontally arranged neocortex, and then into the waiting microtubules of the white matter to shrink water to a small space. PrP is one of the proteins that helped drive this emergent change. PrP is bound to membranes adjacent to the microtubule arrays in neurons. Microtubules are made of tubulin, and they run along the length of the axon and provide the main cytoskeletal “tracks” for transportation. Microtubules also constrain water in tight tubes to create a ton of energy.
Why is this quantum construction critical in the brain?
PHYSICS GEEKS: The natural carriers of the electric currents on the cell membranes of the neocortex, which are laced with DHA and iodine, would act like a magnetic flux tube that also carries electric fields. Remember that a magnetic field is generated perpendicular to an electric current. The electric current in the neocortex runs in 6 longitudinal layers. This means that the magnetic field is perpendicular to the neocortex, which would drive electron flow down to the white matter axon tracts where microtubules and water exist in the brain. A very simple deformation of the imbedding of a constant longitudinal magnetic field also generates a longitudinal electric field. This augments electron currents in the brain. So, a magnetic boost can help electric flow and vice versa. Becker’s work showed these currents at work, he just had no clue where they came from. With a slight generalization by applying the rules of electromagnetism, one obtains helical electric and magnetic fields along the axis of the neocortex and the white matter tracts. The crucial difference is that these currents would be quantized rather than be standard ohmic currents, such as the ones in your house. This allows one to see how length scales of proteins and their currents can alter the biological body or tissues. Proteins are versatile macromolecules which perform a variety of functions by changing their conformational shape. Such functions include muscle movement, membrane firing via openings and closings of protein ion channels, molecular binding, enzyme catalysis, metabolism, movement and phase of cytoplasm. Life is organized by changes in protein shape. Individual proteins are synthesized as linear chains of hundreds of amino acids which “fold” into 3 dimensional conformation. The precise manner of folding for each protein depends on attractive and repellent forces among its various amino acid side groups.
Although complete linear sequences of amino acid chains are known for many proteins, predicting their final 3-dimensional folded shape using computer simulation has proven difficult if not impossible. Perhaps protein folding is a quantum computation tied to the ever changing redox potential of the local environment?
The two main driving force in protein folding occurs as follows:
1. The last four months webinars has shown you how collagen is constructed with the DC electric current to make a triple helix of collagen which is hydrophilic and surrounded by water.
2. The uncharged non-polar groups of particular amino acids join together and avoid water. Repelled by solvent water, “hydrophobic” non-polar groups attract each other (by van der Waals forces) and bury themselves within the protein interior. As a result intra-protein hydrophobic pockets occur, composed of side groups of non-polar (but polarizable by light) amino acids such as leucine, isoleucine, phenylalanine, tryptophan, tyrosine and valine.
These two constructs make different charged plates within the substance of the brain have have different electric and magnetic fields and react differently to polarized light.
All of these currents flow through proteins and water in our brain. As optics change in water, even longer scales become assignable to the magnetic body developed in the brain. This is why the brain’s main chromophobes are Vitamin D and A. They represent the two capacitor plates. This magnetic body developed in the brain can be used to cause distant coherent effects with small fluctuations in energy. This can be due to electron flow or to a magnetic effect. All of these complex physical properties are controlled by the underlying protein interactions at a larger tissue scale. Back to the Non geeks section.
MORE REASONS DARWIN WAS WRONG
Darwinian evolutionary theory cannot explain why sex, aging, or death have evolved. The reasons all three have showed up in eukaryotes is tied to the quantum mechanism detailed in OSF3 and the above thoughts.
Humans have a PrP gene. When knockout mice were created to have their PrP gene removed, they could not develop a prion disease. These mice, however, gave me what I was looking for. One of the original six papers I was given at the beginning of my journey was about Pusiner’s knockout mice. Charles Weissman and Prusiner described the only difference they both could find in these knockout mice after the gene was removed. It was that they were quite normal except for some subtle defects in sleep-wake cycles. This was one of the six papers I was given at the beginning of my transformation. It was a huge clue for me.
Here is where I linked the quantum mechanism to circadian signaling and the optics of the brain. This showed that a new protein is the only canvas that is needed to begin innovating a new evolutionary trajectory. Optics are all about the photoelectric effect. I realized that if optics were active in the brain, then the mechanism had to be quantum, not genetic, in the brain for prion diseases to occur. It also meant that the same thing was true of neocortical development in humans and how the leptin receptor really worked within the hypothalamus.
This insight was shocking to me ten years ago, but it was indeed the data shown to us. Some of us paid attention to the data and not what we were taught about nucleic acids. Guys like Gary Taubes tried like hell to make a name for himself by mocking a physician who found something very unusual and counterintuitive. Just because Prusiner could not explain the mechanism did not mean it was not real. In many ways, Taubes’ antics were very “Ancel Keysesque”. I find that ironic, even today.
What was the key? The normal membrane bound PrP made new proteins that either worked well optically or electronically with DHA and water chemistry in the brain. This makes us super energy efficient because it improved optical energy transduction. When the protein’s bending was altered by a lowered redox potential, it did not work well with DHA. The result was a prion-like disease. Death came fast by quantum design. Today, all autoimmune and neuro-degenerative diseases tie directly back to the failure of this fundamental quantum mechanism.
This quantum mechanism breaks the central dogma paradigm of protein folding.
When you understand the power that this quantum mechanism has to cause every disease that afflicts us, you begin to realize you do not need a new Rx for every disease we face. The quantum theorem fits all situations, and is not a specific Rx for any malady. I showed you how PrP helped form a human brain.
Now I am going to show you how this manifests in a disease that no one can figure out. They can’t figure it out because they are missing this information.
1997 Nobel Prize in Medicine
The Nobel Prize in ’97 was given for the discovery that human proteins devoid of any nucleic acids have caused many diseases that heretofore have been unexplained. These diseases have all occupied the biological wastebasket of diseases with unknown causes for close to 100 years. What the Prize was not given for was how was protein templating causes evolutionary trajectory changes. This is where I entered the game. As a neurosurgeon, I deal with prion diseases, neurodegenerative diseases, and autoimmune diseases frequently. Most people know that neuro-fibrillary tangles are made of amyloid and tau proteins. 30 years of shocking research led the Noble committee to give the 1997 award to one person who basically made the statement that amyloid plaques consisted of protein parts without any nucleic acids, causing disease in humans. These diseases were also found in animals they cultivated. I got interested in his work and looked for clues he might have missed. I did the same thing with Becker, Ling, and Pollack.
It turns out that all amyloid/tau plaques are made of prions (PrPc), which are very small fragments of amino acids that are less than 21 kilodaltons. Dalton (Da) is an alternate name for the atomic mass unit. Kilodaltons = kDa. KDa= 1000 Daltons. Daltons are how we determine the molecular mass of any protein. Average Molecular Weight of a DNA basepair = 660 Daltons. Molecular Weight of double stranded DNA = (# basepairs) x 660. The average molecular weight of an amino acid = 110 Daltons. This has huge implications for mass equivalence and thermodynamics in the brain.
Most MDs are taught that mis-folded proteins are “ancillary inert junk” that shows up on a pathologist’s slide during autopsy. No one has a clue that they are the key thermodynamic metric in why people are developing neuro-degeneration and autoimmune diseases today.
These proteins are altered when they become ionized or deionized by the electromagnetic force. This changes their shape, charge, phenotype, and physiologic function. This phase transition to a oligomer or polymer amyloid protein from the normal PrP protein begins to alter the optics in the brain and in tissues where they form. In OSF 3, and the May 2014 webinar, you learned about optical chromophobes and how they directly change the optics in brain tissue to form “toothpaste or tofu” consistency of the brain parenchyma based upon its energy state.
A chromophore is the part of a protein molecule responsible for its color. You briefly heard about this above. Proteins have some key optical features. The color arises when a molecule absorbs certain wavelengths of visible light and transmits, or reflects, other colors of light. The brain is capable of doing this to all wavelengths of the visible spectrum based upon its variable tissue optics. This means the brain is very sensitive to changes in optics due to protein mis-folding. When a protein mis-folds in the brain, it causes you to concentrate transition metals that induce aging via a change in a process called ubiquination. Ubiquination is the process that removes mis-folded proteins in the brain to improve its optics as damage accumulates under normal function. Ubiquination only happens during a process called autophagy. Autophagy is active during sleep. It is also augmented when a person intermittently fasts or when they exercise, but only when their overall redox potential is good. If the redox potential is not good, the last two methods to augment autophagy do not add to the clearance ability of bent proteins. In fact, they worsen it.
This means that sleep is your critical physiologic mechanism to clear mis-folded proteins. Here is the key point for you all to remember: Autophagy can go on indefinitely if the redox potential stays high. The redox potential is 100% tied to electron collection and ideal optics in the brain. Optimized autophagy is the key to living long and healthy.
The chromophore is a region in the protein molecule where the energy difference between two different molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can be absorbed by exciting an electron from its ground state into a more energetic state. A few examples of a chromophore include Vitamin A, Vitamin D, hemoglobin, bilirubin and amyloid protein. The prion PrPc (27-30kDa) is a common prion protein found in the cell membranes of a healthy brain. It is made up of all 20 amino acids known to exist in nature. Circular dichroism shows that normal PrPc had 43% alpha helical and 3% beta sheet content, whereas PrPsc was only 30% alpha helix and 43% beta sheet. This refolding renders the PrPsc isoform extremely resistant to proteolysis. Here you can see a new property show up in this protein without any input from nucleic acids.
The normal PrPc protein acts as a protein template in this mechanism. This template can be modified by the electromagnetic force in several specific ways to form emergent protein fragments that can cause many of the diseases based upon how the mass, length, or size of the protein is altered. These diseases are ones we all know about, but have no clue where they came from in today’s world. Some examples are MS, ALS, AD, PD, HD, schizophrenia, NF-1, NF-2, Sleep apnea, GBM and many many others………
THE PRION STORY
The story began in the brains of people afflicted with amyloid plaques in scrapie infected brains (PrPsc) in New Guinea. All of these patients’ brains were subsequently found to harbor PrPsc prion proteins. Today, we know all neurodegenerative diseases stem from protein folding abnormalities in the brain. Since prions are proteins, most would guess “good autophagy” could remove them using ubiquination pathways. Mis-folded proteins are normally removed by ubiquination during autophagy, as mentioned above. This normally happens when we sleep. I mentioned above that the PrP knockout mice all showed alterations in sleep and wake cycles. This raises a key point for you to consider. What happens to these mis-folded proteins when we cannot sleep? Consider how Michael Jackson died. He asked for a general anesthetic because he could not sleep. Maybe the King of Pop knew something his doctors did not?
Today, we have many new diseases caused by prions. No one has yet explained how the PrP template mechanistically turns into the PrPsc to my knowledge. I think I can.
Today we have neurodegenerative diseases that contain Lewy bodies, beta amyloid, synaptophysin, Kuru, CJD, and GBM………..etc.
Here is a thread from my forum that will give you a deep clue that I might be correct.
When electrons or photons are lost to the environment outside of a cell, proteins cannot have the proper quanta of energy added or subtracted. This addition or subtraction determines a protein’s physiologic function.
What happens when you lose an electron? You become a positively charged protein and your shape changes!
Prions and chaperone proteins have something in common. Their length and shape are always tied to quanta added or subtracted to them. They are all small proteins made up of alpha and beta sheets. This is part of the secondary protein folding that is determined by the nucleic acid code. But it turns out that secondary structures are altered by things unrelated to the nucleic acid codes. The surrounding cellular or organelle redox potential determines how much of each one is present in certain areas of the cell. This is how conditions of existence affect change at a very fundamental level. The percentage of remaining secondary bending can then be used to determine physiologic function. This is because this relationship alters the shape, mass, and length of the resultant protein. Shape is directly tied to the mass and optics of the tissue in question. This directly links it to a tissue’s energy profile. It can be scaled up or down depending upon the changes in the protein. The shape of prion proteins are dictated where quanta of energy is added or subtracted on the protein. I talked about this 4 years ago!! This links shape to a protein’s thermodynamic profile for mass equivalence.
This change in quantum energy then dictates which amino acid chains were made susceptible or resistant to cleavage by proteases. This is also why people with familial prion disease and sporadic forms also have isolated prions of different sizes and shapes that are resistant to all proteases. What remains obscure to the rest of the world today is how this protein templating occurs. OSF 3 answers that. OSF 4 puts an exclamation point on it. They missed it because none of them realize that the stage that life plays on is the smallest in the universe. It is the subatomic stage. The addition or subtraction of electrons, photons, and protons control this dance.
This is how prion diseases occur. The quantum mechanism of protein evolution in OSF 3 is everywhere you look.
Prion proteins have offended scientists and science writers because they completely break biology’s Central Dogma that nucleic acids dictate change, evolution, and protein creation. Yet again, you see Lady Evolution is not parsimonious. Nature is based upon a quantized foundation.
Amyloid plaques in scrapie and Alzheimer’s were found to be identical in molecular structure, but they caused completely different diseases. This confused neurobiologists and one science writer in particular, Taubes. They were so clueless that they told Dr. Prusiner, who found Lady Evolution’s deep secret, that he was a confabulating liar.
Over the next 20 years Prusiner was shown to be correct. To this day, I know Taubes has no clue what really happens in the mitochondria of the brain or any other tissue with any diet under modern EMF. I believe this because of how he treated the 1997 Nobel Prize winner. I have remained skeptical of his work since. You will need to draw your own conclusions about him from here on in.
Today, biology remains in the dark in regards to why Dr. Prusiner was right. The reason is simple. When the ionization of proteins is altered by quanta energy from the smallest subatomic particles, the energies they carry are transferred to the prion protein to alter its molecular shape by causing them to misfold. This creates a thermodynamic problem for the organism in which it happens.
When energies are altered in proteins, function and phenotype can be altered as well. These changes can be dramatic when there are many subatomic particles involved in the carrying of energy without much mass. A great example of this in action is how apes became human without any major genetic changes in their DNA. I covered that paradox in the brain gut series and I mentioned it in OSF 3 again.
Remember, optics deal with photons, and photons have no mass. They do, however, carry a large quanta of energy. When a protein’s energy is altered for any reason, we see species variation in morphology. This is how natural selection and conditions of existence merge in the new theory of quantum evolutionary biology. This is the smallest stage on which life organizes.
THE UV LIGHT CONNECTION
There was another big clue that people missed with prion disease states. All proteins in solutions absorb ultraviolet light with absorbance maxima at 280 and 200 nm. Protein absorbance ranges are tightly coupled between 260-280 nm. The range of absorbance is directly proportional to cysteine residues. I told you in the April webinar that all 4 collagens rarely have cysteine residues. Now I am going to explain why.
PHYSICAL CHEMISTRY GEEKS:
Why are some proteins excluded from collagen and how many collagens are there?
There are 16 types of collagen.
They differ based upon the amino acids they contain.
The various collagens are distinguished by the ability of their helical and nonhelical regions to associate into fibrils, to form sheets, or to cross-link different collagen types.
Most collagen types in humans are I, II, and III. These normally have very little cysteine within them. A protein’s function is 100% dependent upon tertiary and quaternary protein folding=problems with EMF and sulfur metabolism. All tertiary, and much of the quaternary, protein structure is tied to disulfide bond dependence.
The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mole (251 kJ mol−1). However, they are 40% weaker than C–C and C–H bonds in other amino acids in proteins. The disulfide bond is often the “weak link” in many protein molecules. This allows it to act as the perfect redox sensor of the local environment to dictate physiologic change. Furthermore, this bond is critical in reflecting the polarizability of divalent sulfur. The S–S bond is susceptible to scission by polar reagents (water), namely electrophiles and nucleophiles. Nucleophiles and electrophiles are the official chemistry names for the types of protein species that are considered “electron rich“ and “electron poor”.
Proteins have long been recognized as critical targets of chemicals that produce acute tissue toxicities and cancer. Moreover, the adverse effects of many drugs are frequently mediated via their metabolic activation to reactive electrophilic metabolites, which subsequently bind covalently to proteins. Such reactive metabolites usually have low electron density and react with molecular centers of high electron density (i.e., nucleophiles). Target proteins for adduction usually contain strong nucleophilic sites, including cysteine thiols, lysine amines, histidine imidazoles, and protein N-terminal amines, which are readily attacked by reactive species (Guengerich et al., 2001; Casini et al., 2002). Other proteins contain weaker nucleophilic sites, including methionine sulfur, arginine guanidinium, tyrosine phenols, serine and threonine hydroxyls, and aspartate and glutamate carboxyls.
It’s not an exaggeration to say that nucleophilicity and electrophilicity make up the fundamental basis of chemical reactivity. They are truly the yin and the yang of all aspects of chemistry.
Cysteine acts as a nucleophile in its interaction with a transition metal, such as copper.
RS–SR + Nu− → RS–Nu + RS−
The disulfide bond is about 2.05 Å in length, about 0.5 Å longer than a C–C bond. Rotation about the S–S axis is subject to a low barrier. Disulfides show a distinct preference for dihedral angles approaching 90°. This bond angle is favored when electrons are plentiful within the surrounding environment of the cysteine residue. This means cysteine is more reduced. When the angle approaches 0° or 180°, then the disulfide is a significantly better oxidant.
WHY BAD LIGHT IN YOUR BRAIN CAUSES PRION DISEASES
When photons are powered at 260 nm and strike cysteine residues, they damage the protein more effectively than when they collide with aromatic amino acid residues. Amino acids with aromatic rings are the primary reason for the absorbance peak at 280 nm. Amino acids like tyrosine, tyramine, and tryptophan have aromatic rings. You hear me mention them above in this blog. Peptide bonds are primarily responsible for the peak at 200 nm.
Secondary, tertiary, and quaternary structures of all proteins are all affected by UV light absorbance. When proteins absorb UV light, they transform the electromagnetic spectrum to another form of light. That conversion is usually referred to as infrared light. Water absorbs light best in the infrared spectrum. You might begin to see how things are coupled and quantized now. All proteins are surrounded by water inside a cell by quantum design. Therefore, factors such as pH and ionic strength can also alter the absorbance spectrum of proteins.
The key metric is that the electromagnetic spectrum absorption does not always correspond to the inactivation profile of these proteins. The reason for this should open your eyes wide. When a protein has cysteine residues, the absorption spectra become quite different for the reasons I mentioned above, about cysteine’s disulfide bond angles. This should have told Prusiner that the mechanism for all prion disease was a changing quantum optical protein templating in nature. This implies all prion disease are problems in quantum superposition of proteins. Proteins can be changed optically and electronically. Optics and electronics are the domains of the photoelectric effect in physics, not biology.
People with prion disease tend to have altered their disulfide bonds in cysteine, resulting in less cysteine in PrP. PrP is a very small protein, but it has two cysteines, the rarest amino acid in nature. Moreover, this amino acid acts as a nucleophile when it interacts with copper. This means it donates electrons to copper. PrP’s physiologic function is to allow copper to be absorbed from the extracellular matrix to the interior of the cell. Why is this critically important to understand?
Copper is a highly reactive transition metal that can naturally give or take electrons because of its unusual D shell electrons. This is why copper is found in our most critical enzymes and proteins. Becker found that copper was the doping mechanism in bone that allowed calcium to bind to collagen. Copper is used by carbon in an exquisite quantum dance to form the basic semiconductors in all our cells. All of our semiconductors are carbon based. They use D shell electrons to do the things humans are capable of. But when the redox balance of the equation is off, autophagy becomes less optimized, making you unable to clear these mis-folded proteins. The collateral damage is that you become a transition metal collector in your extracellular space, effectively making you an exquisite EMF antenna. In our native planet this is not a bad thing. In fact, it is what drives evolutionary progress. It is the energy that cause evolutionary motion. In today’s modern wold it is the seat of our decline.
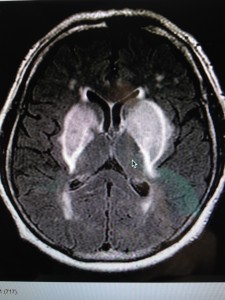
The MRI below shows you a patient with transition metals in their basal ganglia, causing rapid onset of Parkinson’s disease.
You become supersensitive to any EMF just like a star does when it is dying. I covered this in the Energy and Epigenetics series. When an organ collects the critical mass of these metals in our extracellular space, it quickly fails. You begin to die like a star in super nova fashion by some disease process where your “weakest” energy link lurks. The place where your redox potential is lowest is taken out fastest in this process because of the physical chemistry of how transition metals and EMF interact on a quantum level.
The altered disulfide bonds of cysteine then cause mis-folding of normal PrP into a prion that becomes highly infective. Copper movements in the brain become altered and, since this works along cell membranes and synapses, it seems to infect other neurons adjacent to it. This process polymerizes anywhere that PrP is the normal protein. PrP is found in the cell membranes of the human CNS and the human immune system. Every normal mammalian PrP ever studied has two cysteines within its structure. Here is really where neurodegeneration and autoimmunity begin in modern humans. This shows you that food is not a real big part of the equation of either disease origin. It is a factor that determines the severity of the condition based upon how many electrons you eat.
Prion diseases (PrPsc) lose their size, shape, and length relationship because of the failure of the disulfide bond and copper. This alters the mass equivalence relationships in the brain and immune system. This relationship is coupled to a protein’s thermodynamic range. So how does this tie back to the electrons?
Electron Steal Syndrome:
When doped biological semiconductors are joined to metals, to different semiconductors, and to the same semiconductor with different doping, the resulting junction often strips the electron excess or deficiency out from the semiconductor. This depletion region is rectifying (only allowing current to flow in one direction), and used to further shape electrical currents in semiconducting devices. In collagen, we use copper as a doping device. I am showing you how PrP uses copper in the brain and immune system as well. The other two semiconductors in the brain are water and DHA in the cell membrane. Iodine is the doping mechanism ion. If your tissues are collecting transition metals in the extracellular space, then you basically just created a “hole” in your “electron flower pot“. To get your flower to grow, you can add as much water and fertilizer as you want, but if you have 19 holes in your flower pot, your flower will never grow. This is precisely what a loss of electrons causes in our bodies. When we lose electrons, we lose the process of autophagy and we can no longer regenerate our tissues. This is because we cannot clear mis-folded proteins. This is why many people are unaware of how they are constantly losing tons of electrons to their environment even while they are doing a lot of things correctly. Their only clue is that they have poor sleep. Sleep apnea is one of the most sensitive signs.
They have no idea how their environment is creating holes in their semiconductors using non native EMF. Want some more irony? If you listen to the April 2014 webinar, I went over, in detail, how the exact same mechanism of disulfide bond failure happens with copper in bone to cause osteoporosis. Who found this mechanism 60 years ago? Dr. Robert O. Becker MD. Is this a coincidence? Or is it quantum biology in action?
Cysteine is the rarest amino acid and has many electrons to donate or collect bound to its thiol residues based upon the redox of the surrounding environment. Because of this, it is the one amino acid that is an ideal redox sensor. Once again you can see the quantum mechanism at work in prion diseases. When cysteine disulfide bonds are missing or transformed, as seen in prion diseases, beta sheet transformation occurs and a very complex cascade begins in the brain and immune system. When cysteine disulfide bonds are present in the 2 cysteine residues in PrP, we see alpha helices and no disease. In fact, cysteine protease inhibitors prevent PrPsc accumulation in the brain. This is proof that the handling of electrons in cysteine disulfied bonds in relation to copper is critical to maintenance of the proper protein shape and energy profile. The quantum mechanism is the key to understanding how the smallest things lead to the biggest disturbances in a cell.
When our redox potential is suboptimal, proteins (in our cells) collect/store the transition metals which stops/limits the proteins from being hydrophilic. When this connection to water is broken, proteins become unable to fold or unfold correctly, allowing water to bond to the correct side chains of the secondary protein structure. When this occurs, it alters the last two folds of the protein. This action then dehydrates each individual cell and changes its optical density and impairs our collagen function (one of our superconductors) = inflammation (enlarged cell) = hsCRP up……..copper, in this scenario, steals electrons because it is a transition metal that wants to complete its valence electron shell. This action further reduces our redox potential. As the metals collect in us, they begin to interact with excessive EMF and they release more energy in tissues to cause even more tissue damage. This gets transmitted all along white matter tracts in the brain. It spreads like an infection would. The only difference is the immune makers are not elevated because the disease is confined to the brain tissues. As the cascade unfolds, mass increases in neurons as electron density decreases, and the cell loses a lot of energy due to the mass equivalence. This is how mass fundamentally couples to thermodynamics.
Autophagy of defective or incorrectly folded proteins would lead to the buildup of transition metals in our cells. Those increased transition metals would, in turn, attract and amplify non native EMF effects as they do in a star. This would adversely affect other proteins, leading to incorrect shape (which leads to altered physiologic function) and lack of water binding sites.
When a star depletes all its hydrogen, it begins to fuse heavy elements to make sunlight. As the core of the star changes its fuel source, the spectral color of the star also changes the photoelectric effect in its light. In nature, a star explodes when it burns through all of its hydrogen and helium. It then progresses through nuclear fusion turning those gases in its core to other elements. Its core produces energy from hydrogen fusion first, then helium, then carbon, then neon, then oxygen, then silicon, and it all ends in iron. A star accumulates iron as it becomes energy depleted, and it begins to emit large amounts of EMF before it explodes. The EMF it emits interacts with the iron and causes it to explode.
In our bodies, iron reacts with oxygen, resulting in the production of neurotoxic free hydroxyl radicals (Fenton Reactions), which are responsible for membrane lipid peroxidation and accumulation of lipofuscin in neurons. Why do you think iron and copper accumulates in neurons when they are sick and dying? The reason is quantum. The mechanism scales from a star to your neurons and immune cells.
Stars, like humans, also have a life cycle. Very few people see the fractal pattern of human life in a star’s life, but nature does.
Ironically, when I realized this, I immediately solved another long standing puzzle in biology. Every amino acid (except glycine) can occur in two isomeric forms. This is because of the possibility of forming two different enantiomers (stereoisomers) around the central carbon atom. By convention, these are called L- and D- isomers. This is analogous to amino acids having left-handed and right-handed configurations.
Only L-amino acids are manufactured in cells and incorporated into proteins.
Some D-amino acids are found in the cell walls of bacteria, but not in bacterial proteins. Glycine, the simplest amino acid, has no enantiomers because it has two hydrogen atoms attached to the central carbon atom. Only when all four attachments are different can enantiomers occur.
Optical isomers are also designated with the letters (D) and (L) to indicate the absolute configuration. In an absolute configuration, the position of the groups is compared to a commonly agreed upon standard molecule. In amino acids, this molecule is serine. For carbohydrates, it is glyceraldehyde. The capital (D) and (L) designations do not necessarily agree with the (d) or (l) designations, which are based upon the actual rotation of polarized light.
OPTICS AND CYSTEINE
The reason life only uses L-amino acids is the same reason why prions need cysteine to remain normally shaped. Optics. Prion shape is altered by electrons and photons. It turns out that optics are pretty damn important in the proteins of life. All tissues work optically and electronically and these changes dictate the biological processes that underpin life. For my members, you all heard about just how important optics are in the human brain during the May 2014 webinar. The human brain generates light photons (red) from electrons. DHA captures these electrons in the cell membranes of neurons, very close to where PrP is found. This means any alteration in the photo-optics has huge energy consequences in neurons. It turns out that beta sheet prions cause an unusual birefringence pattern in the brain when it is examined by polarized light. We will get to that later.
Optically active compounds exist in two isomeric forms. This is called circular dichroism. The isomer that rotates the plane of polarized light to the left (counterclockwise) is called levorotatory (l). The other isomer that rotates the light to the right (clockwise) is called dextrorotatory (d). The optical isomers are mirror images of each other. The isomers result from the tetrahedral geometry around the chiral carbon center. As a consequence of protein circular dichroism, the secondary protein structure (coded for by DNA) will also impart a distinct circular dichroism (CD) to its respective molecules. This is akin to the spectral lines emitted in a star. Remember the red giant example I told you to hold onto in OSF 3?
Therefore, the alpha helix of proteins and the double helix of nucleic acids have CD spectral signatures that are representative of their structures.
PHYSICS GEEKS: The far-UV CD spectrum of proteins reveals important characteristics of their secondary structure. CD spectra can be readily used to estimate the fraction of a molecule that is in the alpha-helix conformation, the beta-sheet conformation, the beta-turn conformation, or some other (e.g. random coil) conformation. These fractional assignments place important constraints on the possible secondary conformations that the protein achieve. In general, CD cannot say where the alpha helices are located within the molecule and can completely predict how many there are.
The near-UV CD spectrum (>250 nm) of proteins provides information on the tertiary structure. The signals obtained in the 250–300 nm region are due to the absorption, dipole orientation, and the nature of the surrounding environment of the phenylalanine, tyrosine, cysteine (or S-S disulfide bridges), and tryptophan amino acids. Unlike far-UV CD, the near-UV CD spectrum cannot be assigned to any particular 3D structure. Rather, near-UV CD spectra provide structural information on the nature of the prosthetic groups in proteins, e.g., the heme groups in hemoglobin and cytochrome c. This tells us information about how electron addition or subtraction to these proteins alters their physiologic function.
Visible CD spectroscopy is a very powerful technique to study metal–protein interactions. It can also resolve individual d–d electronic transitions as separate bands. CD spectra in the visible light region are only produced when a metal ion is in a chiral environment. Therefore, free metal ions in solutions are not detected. Most people in alternative medicine circles blame heavy metals for a lot of things because they do not understand that the metal-protein interface is the real quantum issue. Removing metals from your body is not the key. Getting them to do what they are designed to do is.
Visible CD spectroscopy has the advantage of only observing the protein-bound metal, so pH dependence and stoichiometries are readily obtained. Optical activity in transition metal ion complexes have been attributed to configurational, conformational, and local environmental effects. Klewpatinond and Viles (2007) have produced a set of empirical rules for predicting the appearance of visible CD spectra for Cu2+ and Ni2+ square-planar complexes involving histidine and main-chain coordination.
CD gives less specific structural information than X-ray crystallography and protein NMR spectroscopy. Those both give atomic resolution data. However, CD spectroscopy is a quick method that does not require large amounts of proteins or extensive data processing. This means CD can be used to survey a large number of solvent conditions, varying temperatures, pH, salinity, and the presence of various cofactors. This makes it ideal for biological assays.
CD spectroscopy is usually used to study proteins in solutions, and, thus, it complements methods that study the solid state. Semiconduction is a branch of solid state physics. I should also point out that this is also a limitation of the technique in the brain because of the DHA lipid rafts. In neuron cell membranes, many proteins are embedded in DHA laden membranes in their native state, and solutions containing membrane structures are often strongly scattered when they are examined. CD is sometimes measured in thin films, and, since the brain is based upon topologic insulation, I believe it may one day have significant clinical utility once quantum medicine is accepted by healthcare.
Topologic Insulators in your brain?
WHY ARE ISOMERS A BIG DEAL FOR LIFE?
L-Alanine is a representative amino acid that is used in human and animal metabolism. The D isomer is not used.
In the metabolism of carbohydrates, only the D isomer is utilized and not the L isomer. Here you see proteins and carbohydrates act quite differently in an optical sense. The brain takes huge advantage of this. This advantage becomes a problem when we deal with PrPsc.
Still think this is not important? Consider the mechanism of Advil. Advil is the drug Ibuprofen. It is sold as a mixture of isomers, although the (S) isomer is most effective and the (R) isomer is also utilized after conversion to the (S) isomer in the body. Your body pays attention to optics and electronic induction even though your biology texts and your doctor don’t. It is time you do, too. Physics dictates biology at all scales.
Life and health are not practical or parsimonious, so Dr. Prusiner’s work fits in perfectly with a quantized mechanism. His theories do not follow Occam’s razor theorem of simplicity, and this is why biologists have argued with him and why guys like Taubes still do not accept his work.
Prions are mechanistically quantum, but Prusiner has not said this himself. I am saying it for him in this blog.
The electromagnetic force is the conductor that makes these prions play life’s music with the small additions or subtractions of quanta energies. It is where form meets ultimate physiologic function. Once these proteins are transformed, so are their physiologic capabilities.
This ends part one of the prion story……….OSF 5 will deal with part 2. It will discuss why mental illness, autoimmunity, and neuro-degeneration begins and why it is exploding.
CITES
DebBurman, S., Raymond, G., Caughey, B., and S. Lindquist. 1997. “Chaperone-supervised conversion of prion protein to its protease-resistant form.” Proceedings of the National Academy of Science USA. 94: 13938-13943.
Haire, L.F., Whyte, S., Vasisht, N., Gill, A., Verma, C., Dodson, E., Dodson, G., and P. Bayley. 2004. Journal of Molecular Biology. 336: 1175-1183.
Jones, C. E., Abdelraheim, S., Brown, D., and J.H. Viles. 2004. “Preferential Cu 2+ Coordination by His 96 and His 111 Induces B-Sheet Formation in the Unstructured Amyloidogenic Region of the Prion Protein.” Journal of Biological Chemistry. 279(31): 32018-32027.
Kaneko, K., Zulianello, L., Scott, M., Cooper, C., Wallace, A., James, T., Cohen, F., and S. Prusiner. 1997. “Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation.” Proceedings of the National Academy of Science. 94: 10069-10074.
Layne, E. Spectrophotometric and Turbidimetric Methods for Measuring Proteins. Methods in Enzymology 3: 447-455. 1957.
Stoscheck, CM. Quantitation of Protein. Methods in Enzymology 182: 50-69. 1990.
Knaus, K.J., Morilla, M., Swietnicki, W., Malone, M., Surewicz, W., and V. Yee. 2001. “Crystal Structure of the human prion protein reveals a mechanism for oligomerization.” Nature Structural Biology. 8: 770-774.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2850414/
Guyer RL. Prions: Puzzling Infectious Proteins. NIH – Research in the News. (http://science-education.nih.gov/nihHTML/ose/snapshots/multimedia/ritn/prions/prions1.html)
Taubes G. The Game of the Name is Fame. But is it Science? Discover. December, 1986. Reprinted at: http://www.slate.com/id/2096/sidebar/42786/.
Prusiner SB. (1995).The Prion Diseases. Scientific American. (http://www.sciam.com/article.cfm?articleID=0009FD80-C3C6-1C5A-B882809EC588ED9F&pageNumber=1&catID=2)
Whitmore L, Wallace BA (2008). “Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases”. Biopolymers 89 (5): 392–400. doi:10.1002/bip.20853. PMID 17896349.
Greenfield NJ (2006). “Using circular dichroism spectra to estimate protein secondary structure”. Nature protocols 1 (6): 2876–90. doi:10.1038/nprot.2006.202. PMC 2728378. PMID 17406547.Prusiner SB. (1982). Novel proteinaceous infectious particles cause scrapie. Science. 9;216(4542):136-44.
Legname G, Baskakov IV, Nguyen HB, et al. (2004). Synthetic Mammalian Prions. Science. 7;305:673-676.
Johnston N. (2004). Clearing Hurdles: Prions Know How to Do It. The Scientist. 18(11):18. (http://www.the-scientist.com/yr2004/jun/feature_040607.html)
Bastian FO, Foster JW. Spiroplasma SP. (2001). 16S rDNA in Creutzfeldt-Jakob disease and scrapie as shown by PCR AND DNA sequence analysis. J Neuropathol Exp Neurol. 60:613-620.
Aguzzi A, Polymenidou M. (2004). Mammalian Prion Biology: One Century of Evolving Concepts. Cell. 116(2):313-327. (http://www.sciencedirect.com/science/article/B6WSN-4BJK582-H/2/70143387edac888b362f04571b6a7988)