suchTHE BLOG TAKE HOME: Since Becker established that human bone uses P/N junctions for semi conductive circuits that regenerate human bone there have been many other ways light’s non linear physical abilities are used in a cell. Could biologic semiconductors be used to make cellular lasers while we sleep to regenerate us? This blog intimately is tied to the April 2016- Sept 2016 webinars and will be particularly helpful for members of this site who have had access to them.
DAY/NIGHT = TRANSPARENCY/OPAQUENESS
Daytime is associated with the DC electric current. At nighttime it goes away. What happens in between is why evolution invented sleep. When electric resistance is increased light emission occurs photoelectrically. This shows you that a DC electric current can be changed to a photonic signal just by a temperature change. Semiconductors lie in an area between the metals and non-metals on the periodic table. Life is carbon based. Carbon is in Group IV of the periodic table.

For the Group IV semiconductors such as collagen, diamond, silicon, germanium, silicon carbide, and silicon germanium, the most common dopants are acceptors from Group III or donors from Group V elements. Boron, arsenic, phosphorus, and occasionally gallium are used to dope silicon.

By doping carbon based semiconductors with Group V elements like nitrogen and phosphorus, extra valence electrons are added that become unbonded from individual atoms and allow the compound to be an electrically conductive N-type semiconductor. This is why carbon based collagen is the number one protein in all living cells and it is also why phosphorus is mission critical in turning on and turning off physiologic pathways used inside of all living cells. This is also why Nature has put chlorophyll and hemoglobin put nitrogen next to carbon and transition metals.
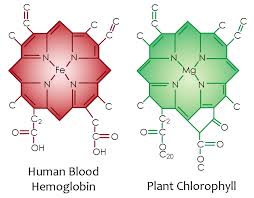
N-type semiconductors have a larger electron concentration than hole concentration. The phrase ‘n-type’ comes from the negative charge of the electron. In n-type semiconductors, electrons are the majority carriers and holes are the minority carriers. N-type semiconductors are created by doping an intrinsic semiconductor with donor impurities (or doping a p-type semiconductor as done in the making of CMOS chips). A common dopant for n-type silicon in technology is phosphorus. Life does the same thing, but it pairs carbon with nitrogen and phosphorus in its semiconductive circuits. Physics geeks will recall in an N-type semiconductor, the Fermi level is greater than that of the intrinsic semiconductor and lies closer to the conduction band than the valence band.
In life, I have had a sense for a long time that carbon based semiconductors have used nitrogen, phosphorus, sulfur, and the transition metals as the key “doping atoms” inside of cells because they are found in all key proteins and in mitochondria and blood. Every cytochrome mouth has an iron sulfur couple in them for a reason. In fact, Becker found bone collagen is an N-type semiconductor and it is also piezoelectric. Piezoelectric materials are able to turn mechanical energy into electric energy. Recall that vibrations, oscillations, and solitons are a form of mechanical energy. I told you in Time 20 that the 100 Hz vibration was key in fat burning. In bone, the N type junction is collagen and the P-type junction is apatite. All biologic semiconductors, that use a P and N junction also are fully capable of emitting light. Becker found bone was a light emitting diode (LED). Becker discovered this in his experiments by looking at currents flowing “uphill”. This uphill current is is called a reverse bias current. It is used to look for photoelectric effects in circuits. Many semiconductors absorb energy from light, and any current flowing through the material gets a boost. In these experiments Becker did on bone, just shining light on the bone elicited an increase in current. The light bone emitted was infrared. This makes sense when one considers that bone is surrounded by water, contains massive amounts of water, and its bone marrow is filled with blood plasma which is 93% water by volume. Becker knew most semiconductors absorb UV light and fluoresced as a result. The light semiconductors emitted was almost always a lower frequency so the finding that whole bone emitted infrared light intuitively told him bone had to absorb UV light. He found whole bone fluoresced a bluish ivory, while collagen yielded an intense blue light, and apatite emitted a brick red color.
Bone also has a dopant, two copper atoms, and these atoms rectify the current. Rectifying a current gives it a direction. This makes the bone semiconductor circuit sensitive to current’s direction. A current’s direction is critical in how light can or cannot be polarized. Sunlight is unpolarized and man made light is polarized. Sunlight is designed to be polarized by cells when they are transparent to the sun’s light during daytime.
The cellular uses of the photoelectric effect is important to understand totally to understand what it is capable of doing to cell physiology. The details are critical. Wherever light goes, the electric and magnetic fields are disturbed perpendicular to the direction of propagation. This propagating disturbance is what makes light a wave. The fact that it disturbs these fields at right angles to the direction of propagation makes light a transverse wave. Malus and Thomas Young used this to refute Newton’s ideas on light in 1801.
Why is this non linear detail of light important? It shows you why the eye and the skin are radically different surfaces in biology. Imagine a light wave traveling toward you from the sun, on its way to entering your eye and retina. In what direction is the electric field vibrating? This question is important because light is both electric and magnetic as it travels, but it is usually the electric field that we are interested in discussing polarization. Is the electric field up and down? Is it left and right? All orientations are plausible because both alignments are perpendicular to the direction of the propagation of the wave from the sun. Sunlight sources are unpolarized. The electric field is vibrating in many directions all at once; the one caveat, is they are all perpendicular to the direction of propagation.

Polarized light is unique in that it vibrates mostly in one direction. So controlling the direction of vibration is critical in cells being able to use the non linear effects of polarized light. This is critical when you realize that D-glucose is used by life, L-fructose is used by life and that all proteins are left handed from a chiral perspective. Food guru’s do not understand these optical features of steroechemistry link to light controls for all of biochemistry. The dopants in a semiconductors inside of cells give cells a quantized level of control of the 100,000 substrates of biochemistry. Any direction is possible as long as it’s perpendicular to the direction of propagation as the picture above shows.
Is how cells produce polarized light critical to physiologic function? Yes. Is their method similar to how man made devices produce polarized light? The short answer is no, cells and technology polarize light very differently. Sunlight and water are the keys in cells.
Sunlight can be polarized by reflection from a dielectric surface. EZ water has a high dielectric constant of 160 and acts like a molecular mirror. HYPERLINK
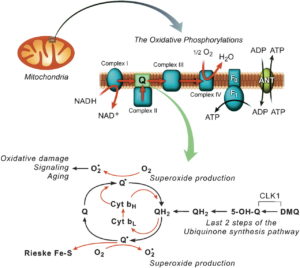
The glare off a mirror is reflected light that partially polarized at right angles to the plane of incidence. This is called the Brewster angle. This angle is made when reflected light is completely polarized when the reflected ray is perpendicular to the refracted ray. This means that the Brewster angle shows us that the tangent of the Brewster angle equals the index of refraction of the medium. Water has been shown to have a different index of refraction than bulk water by Pollack et al. experiments. Scattering of sunlight also can polarize sunlight. This happens in our atmosphere. This is why the sky is blue and why rainbow’s are visible when water scatters light in rainy environments. Light scattered from small molecules is polarized at right angles to the direction of propagation of the original beam. Dichroic crystals can also polarize light. Dichroism is a physical property of a crystal that presents different colors by transmitted light, when viewed in two different directions, the colors being unlike in the direction of unlike or unequal axes. This absorbs the component of wave polarized in a particular direction. What type of things use this mechanism? Tourmaline, ubiquinone, quinine iodosulfate in viscous plastic — crystals oriented by extrusion and giant thin crystals of iodosulfate of quinine Herapathite: the sulfate of iodoquinine discovered by Edwin Land (1909–1991) United States. Ubiquinone and Ubiquinol are USED in the Q-cycle of the mitochondria that connect cytochrome 1, to 3, and 4. Cytochrome 4 is the heme cytochrome c oxidase. Did you know that mitochondria can polarize light in dichroic fashion and this is key in cytochrome signaling? HYPERLINK.
The last way to polarize light is by using birefringent crystals like exclusion zone (EZ) of water, calcite (Iceland spar). Icelandic spar was the crystal that discovered optics for humanity by Malus.
These type of crystals have a preferential direction of polarized and propagation “o” ray and “e” ray (ordinary and extraordinary). Icelandic spar’s optics, shown above, flummoxed Newton and caused Malus and Thomas Young to reject Newton’s ideas that light was a particle force only. Icelandic spar was used by Vikings to navigate the seas during cloudy days and night because of its ability to polarize light even when the sun was absent. Might this effect be beneficial to cells on cloudy days and night time during sleep? Yes it is. It always told them the correct orientation toward the sun whether they could see it or not. Birefringence is a non linear aspect of light that can be seen in human cells when you look at them under a polarized light microscope. The reason this occurs is that EZ water is fully capable of polarizing incident sunlight because EZ water is a anisotropic liquid crystal.

Does anyone want to venture a guess what this is? This is where the Bering Sea meets the Alaskan Gulf. This is two bodies of water with radically different chemistry and different light frequencies in each because of their high latitudes. The water forms an exclusion zone and the result you see here is where two bodies of water that have markedly different EZ’s where they meet in open water.
WHAT DO SEMICONDUCTORS DO TO POLARIZED LIGHT?
Semiconductors change the direction of light’s electric field to alter the polarization of light to optimized cell function. Since cells are filled with proteins, do proteins have some specific stereochemistry associated with them to take advantage of polarized light? Aren’t all amino acids chiral? Yes they are. Isn’t one achiral? Yes, glycine is achiral. Could this be how the quantum mechanism of evolution works that I mentioned in the OSF 3 blog?
Given what I said above about Icelandic spar, here is a thought to ponder: Do you think you know all about sunlight and how it interacts with cells? Could polarization be our 6th sense found in our mitochondria that acts just like the “pupil” in your eye? Let me refocus your perspective now. Remember the pupil is the only known true perfect blackbox radiator in humans. I have a sense that polarized light around mitochondria is its version of a pupil so a mitochondria can sense light optically and magnetically to harvest its energy and information for the mitochondria to communicate to it the critical information about the exterior environment. If you think this is foolish I will remind you of the basics of all wireless communication systems is simple. All it takes is a coil (mitochondria) coupled to a capacitor (EZ) resonating at a specific resonance frequency to communicate in wireless fashion. We know from Dr. Doug Wallace that mitochondria that fat burn have to oscillate at 100 Hz. Incident sunlight is unpolarized and it is polarized by many things in cells that are semiconductors like EZ water. The ways in which it physically happens is mentioned above. Is there an animal model we could look at that uses this mechanism? Marine animals actually have photoreceptors that react to a specific type of polarized light. These animals live in the ocean. How does the ocean effect sunlight?
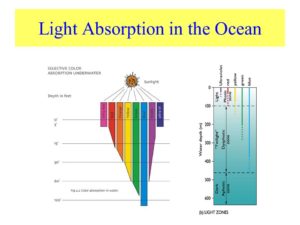
Might sunlight be affect by EZ water in cells the same way it is in the ocean? If so, could the effect on fish in the sea teach us about light from the sun would affect our mitochondria? Remember mitochondria is swimming in a sea of EZ inside every cell. Since UV light is not absorbed well at cell surfaces that the sun interacts with (see skin below), marine animals tend to react to polarized light at frequencies above 450nm which is in the blue range of light. Blue light penetrates deeply into tissues with water.
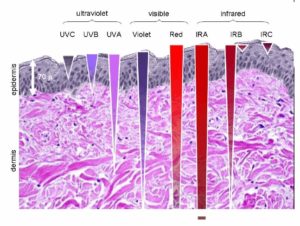
If the animal lives in air, like man does, polarization can happen at lower frequencies than marine animals, therefore it can dip deep into the UV range. NADH is a fluorophore protein at reacts at 340 nm. Did you know that insects and bats react to polarized light in the UV range? Did you know that fish, cephalopods, and crustaceans react to polarized light in the blue range? The reason why is in this picture below.
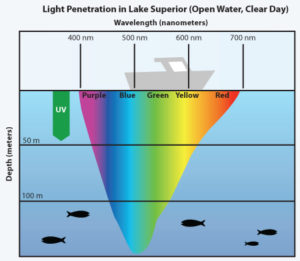
Note that blue light penetrates deeply into the photic zone in sea water to affect marine photoreceptors and mitochondria.
Why am I going down this path for you now? In my April 2016 webinar Q & A, I told members that cells use every non linear aspect of light physics has found to harvest energy, information and data from sunlight. Might the polarization mechanism of light be important in your cells in way you’ve never considered? Since mitochondria are organelles suspended in a sea cytosolic EZ water might the mechanism react in our cells as it does in marine animals? Might our organelles have some type of polarization photoreceptor close to mitochondria that responds and reacts to light differently than it could at the sun-skin interface where the circulatory system comes to the surface to meet light? I told you above the entire Q cycle in the mitochondrial cytochromes act as a giant polarizer of light. Did you also know polarization of light in water is surprisingly insensitive to the incident wavelength of light? Patterns of both the polarization of light in water reveal an overall e-vector orientation and degree of polarization that are remarkably similar from 360 to 550 nm. It turns out that light in the ultraviolet range is “slightly less polarized” than at wavelengths beyond 400nm. This small difference can lead to major changes because cells are a non linear receptor for light. This means small incident effects can lead to massive changes in output. Might this be why NADH accepts excited electrons at 340 nm light? Remember electrons that enter cytochrome one are excited by the sun generally grow in long light cycles. This is why carbohydrate electrons enter ECT at the NADH/NAD+ cytochrome 1. Is this why nature built a mitochondria to funnel these electrons from cytochrome 1 to cytochrome 3 and 4 via their connection to the Q cycle? I want you to also remember that all cells are known to release ELF -UV light for signaling. Under duress, they release even more of this light. Light waves move or propagate through atoms in the respiratory proteins by moving charges. This is why the Q-cycle is mission critical in mitochondria Why did Nature build us this way? Might this light frequency be linked to the polarization effect mentioned above? Is this how UV light that excited electrons in carbohydrate are muted physically in some way with respect to cell water so that they don’t short out cytochrome 1? Might this muting of polarization be linked to the oscillation frequency of the inner mitochondrial membrane to perform beta-oxidation in the cytosol of cells? I think so. Light wavelengths change has they move through the Q-cycle. This means UV light that excited electrons at cytochrome one can wind up a red light the emerges at the end of the Q-cycle to condense cytochrome c and improve the rotating head of the ATPase. Spectral shifts of light also occur at infrared frequencies. This is why mammals are warm blooded. Here is an example: When sunlight strikes your house, the house absorbs that light and the house re-radiates some of that energy to warm the house. Mammals do the same thing with their huge mitochondrial capacities. So do birds. Is this why the EZ cell water has a higher refractive index than bulk water? EZ water is at 270 nm and this is 10% higher than bulk water. We see this effect in MRI’s on diffusion weighted imaging. This higher refraction index means that EZ water’s light absorption spectra will radically differ in the UV-light visible range and in the infrared range. How do all these things fit in the quantum engineering of cells using semiconduction? Do mitochondria generate magnets fields? They do. Do you know anything about the Faraday effect?
The Faraday effect of light generates massive magnetic fields around these light bundles. Mitochondria are known to generate massive magnetic fields from MEG data we get in medicine. I am so interested in this effect because I think it maybe the basis of why mitochondria might be the place in a cell that harbors and hides a transient magnetic monopole in cells. The Faraday effect is an optical magnetic effect that occurs in most optically transparent dielectric materials including liquids like water. A dielectric is an electrical insulator that can be polarized. When a dielectric (EZ) is placed in an electric field, electric charges do not flow through the material as they do in a conductor. Instead, they slightly shift creating mpre positives on one side of the dielectric and more negatives on the other. Normal tap water has a high dielectric constant of 80, but EZ water inside a cell is 160. This is how the massive electric fields in a mitochondria are shielded while magnetic fields are uneffected. This becomes important in changing the optics of tissues by altering their transparency. This is why I mentioned the Brewster angle earlier. During the daytime when the sun is shining, cells are made transparent by UV light exposure on the skin and the subsequent presence of the DC electric current in cells. For the Faraday effect’s magnetic/optical properties to manifest, a local magnetic field (mitochondria) must influence the crystal or liquid crystal dielectric under the influence of this magnetic field. This occurs in the vicinity of the mitochondria in every cell. The Faraday effect causes a rotation of the plane of polarization within a media/tissue which is linearly proportional to the component of the magnetic field in the direction of propagation. This tells us the charge within and the ability to polarize light by a mitochondria would be a requirement of the system in a cell. Do we have evidence of this? Today we know that K+ ions can polarize light in cells to make them birefrigent under a polarized light microscope. Other reasons I am so interested in this recent finding is that the Faraday effect is used in spintronics for topologic insulator research to study the polarization of electron spins in semiconductors. These things also occur in mitochondria because electron spins are altered to make free radical chemicals in the cytochromes. Faraday rotators can be used for amplitude modulation of light and are the basis of optical isolators and optical circulators; such components are required in optical telecommunications and other laser applications. My members will really perk up with this last physical fact because my August 2016 webinar was linked to how cells make laser at night time when sunlight is absent. This effect has huge implications for biochemical control using entangelment and how memories are formed and recalled using holography in the EZ crystal of cells.
I am describing an optical magnetic communication system in cells in this blog? I am.
How might this system interface with substates found in a cell to work?
Stereochemistry of proteins and amino acids are the short answer.
All sugars are “right-handed”, but may rotate the polarization of light in either direction: DNA and RNA use sugars for this reason to encode data from polarized light it gets from mitochondria. Since mitochondria are diachronic they mimic Icelandic spar and properly directly light when a cell is transparent or opaque based upon the day/night cycle.
The coding = right/clockwise/dextrorotatory and left/counterclockwise/levorotatory.
Determining whether a particular compound is right or left handed is determined by a particularly complicated set of rules in chemistry that are not important to understand now.
The term chiral in general is used to describe an object that is non-superposable on its mirror image. Chirality: right-handed sugars and left-handed proteins are what fills out cells. All amino acids except glycine, are chiral and exist naturally. All the other isomers may be made indigestible, and hence may cause diarrhea, may be toxic, some organic compounds exist in both forms e.g., carvone (a diterpene) has two enantiomers: D-(+)-carvone which is found in the seed oils of caraway, dill, and anise; and L-(-)-carvone which is found in spearmint oil.
Glucose made by the sun via photosynthesis and the TCA cycle by unpolarized surface sunlight is always dextrorotatory, also called dextrose, α-D-(+)-glucose (C6H12O6 six-sided ring). L-Glucose is indistinguishable in taste from D-glucose, but cannot be used by living organisms as source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway. This tells us that hexokinase is also optically active in the mitochondrial matrix where glycolytic metabolism occurs.
Fructose is levorotatory, also called levulose, α-D-(-)-fructose (C6H12O6 five-sided ring). This makes it optically different from D-glucose for deep reasons in liver metabolism. All 20 amino acids but one (glycine) are optically active chiral molecules. This means they all interact with polarized light to exchange information before they are placed into their final protein form. Once they are incorporated to proteins and they undergo the tertiary and quaternary bends, all proteins become left handed with respect to polarized light because of the direction of the electric fields in cells that are dictated by their semiconductors in cells. If this mechanism breaks down prion like diseases and neurodegeneration become more likely. This is how cell photonics connect and direct proteins to communicate with EZ water, mitochondria, and the nuclear genome.
For example, sucrose is a disaccharide of glucose and fructose, sucrose is dextrorotatory, when the two molecules are separated (hydrolyzed) the rotation caused by the fructose dominates making the mixture levorotatory, thus the optical polarization of the solution has been “inverted”. The sugars themselves have not had their individual chirality inverted. This would require inversion of the molecule in 3 separate places. This unique optical feature imparts different physiologic effects in different cells because of how mitochondria are tuned by light.
HOW ARE MITOCHONDRIA OPTICALLY TUNED?
Thus, there is no particular optimum wavelength for photoreceptors that are polarization specialists, but the best signal-to-noise ratios will exist at wavelengths at which light is brightest. For us, animals on land, that is in the UV range. UV light has many non linear properties that blue light and red light do not. Consider ocean dwellers: For marine animals it is in the blue range because UV light cannot penetrate the surface of water well. Might this quality in the ocean be true for mitochondria in your cells because mitochondria in you are swimming in a sea of EZ in your cytosol? If this true, might evolution be using mitochondria as a polarizer sitting in a cytosolic sea of EZ water? Would she have built in a “type of polarization” into her photoreceptor design so that the alterations in their size, shape, and distance between the respiratory proteins act as a tunable polarizer to interact with a varying magnetic field from a mitochondria? Yes I think she did.
Recall that a mitochondria that can fat burn optimally needs to oscillate at 100Hz. This vibration would link to a process that makes polarization have to be precise. Nothing is more precise than quantized polarization. Why? It is based upon light frequencies associated with excited electrons delivered to ECT in mitochondria. The ECT contain 5 cytochromes and within ECT their is a quinone cycle. Quinones can polarize light as mentioned above, because of the Q-cycle handles electron tunneling from cytochrome 1,3, and 4. Might this polarization tuning effect be able to alter the spin of electrons? Is this how a mitochondria tunes electrons to make free radical signals? I think so. Would the atomic arrangement in cytochromes be inherited from the maternal side so that it would be optimized to match the predominant wavelengths downwelling light of the environment the animal exists in? I think so. Might this atomic arrangement be the key anatomy of what makes mitochondrial haplotypes unique to environmental light exposure so that they must link to specific polarization effects? Yes, I think nature is this shrewd.
Moreover, should we expect that the middle-wavelength polarization receptors of marine invertebrates are spectrally well placed if this mechanism I am describing is present in living things? They are present in fish. their photoreceptors and cytochromes have been found to be atomically built to act in the blue and red range of light. Why is that? That is the type of light that penetrates deeply into water in the photic zone. Hey, don’t mitochondria release light above 500 nm too? Yes they do. Is this coincidence or quantum engineering 101 because of how solar radiations differ from the ones technology devices?
Why would polarization of sunlight be mission critical to cell communication and mitochondrial medicine? Polarized light carries information about magnetic and electric fields of its environment. A mitochondria generates these fields and light can carry this information through cells using Fresnel and Snell’s law of least time principle. I expect soon we will find that all ELF-UV light cells released from cells has much of their data buried in it via frequency spectrum and its polarization. I told you in the OSF series I felt polarization defects of proteins lead to all prion and neurodegenerative diseases. Now I am giving you the quantized mechanism of how I think it happens. I see what few others do because i understand the physics of how organisms are built. Polarized light carries the data of magnetic fields, chemical interactions, crystal structures, quality variations, and mechanical stresses because all of them can all of these physical effects affect the polarization of a beam of light through a tissue with these variations. Nature is slicker than anyone imagines. The use of non linear aspects of light should astound you. We need to assimilate light from outside and then use it to program water and proteins to allow life to manifest. The captured light is slowed down and this allows time to manifest. The capture light is then reused and it begins to program amino acids and proteins. This light can be change to a DC electric current and we can re-make light from cells at night when they are opaque by using we semiconductors. These semiconductors acts as LED’s are Becker has proved. In fact, every living cell is loaded with them because of this reason.
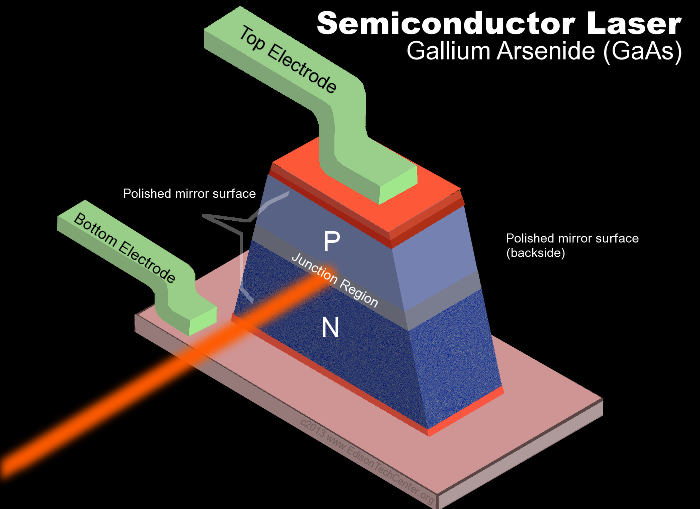
The P-type is the positive side of the semiconductor and the N-type the negative side. P-N semiconductors that emit light are also called light emitting diode (LED’s). A light-emitting diode (LED) is a semiconductor device that emits visible light when an electric current passes through it. The light does not have to be particularly bright, but in most LED’s it is monochromatic, occurring at a single wavelength. The output emission from an LED can range from red (at a wavelength of approximately 700 nanometers) to blue-violet (about 260 nanometers). Some LED’s emit infrared (IR) energy (830 nanometers or longer); such a device is known as an infrared-emitting diode (IRED).
An LED or IRED consists of two elements (molecules or atoms) of processed material called P-type semiconductors and N-type semiconductors. In bone, collagen (N) and apatite (P) are those two materials that create light in periosteum. These two elements are placed in direct contact, forming a region called the P-N junction. In this respect, the LED or IRED resembles most other diode types, but there are important differences. The LED or IRED has a transparent package, allowing visible or IR energy to pass through. Also, the LED or IRED has a large PN-junction area whose shape is tailored to the application. Benefits of LEDs and IREDs, compared with incandescent and fluorescent illuminating devices, include: Low power requirement: Most types can be operated with battery power supplies. High efficiency: Most of the power supplied to an LED or IRED is converted into radiation in the desired form, with minimal heat production.
The LED is a light source which uses semiconductors and electroluminescence to create light. There are two major kinds of light emitting diodes: LED and OLED. The LED is different than EL lamp in that it uses a small semiconductor crystal with reflectors and other parts to make the light brighter and focused into a single point. The OLED is very similar to the EL lamp in design, using a flat sandwich of materials. It is different than the LED and EL lamp in that it uses organic (carbon) molecules in the layer that emits light.
Semiconductors in technology are sensitive to being damaged by heat, so large heat sinks must be employed to keep powerful arrays cool, sometimes a fan is required in gadgets. Humans don’t need them because we use EZ water as our heat sink. This is why all biologic semiconductors find them selves next to water. Water is the ideal chromophore to absorb this heat. Heat = IR light. This explains why dehydration from any cause is so damaging to biologic semiconduction. This causes a massive cost in energy efficiency because it greatly reduces the energy efficient advantage of light emission. Less light emission is due to a small rise in temperature would affect the polarization of light.
In LED’s when incident light hits it, and small DC current is measurable. This occurs in daylight for carbon based semiconductors. At night time, when incident sunlight is absent, the temperature drops and more light is emitted from cells while the surfaces remain opaque to light. UV light makes things transparent and the lack of UV light makes things opaque. This explains fully the quantum purpose of sleep. The physical requirements of semiconduction and PN junction set the standards of when sleep and regeneration can occur. Becker found that all plants and animals can regenerate by using DC electric currents. The presence or absence of the DC electric current determines when light emission can occur. During the day the DC current is present. At night it is absent.
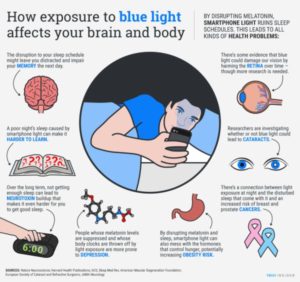
Blue light at night destroys these light relationships to the DC electric current presence or absence and this is why it disrupts sleep and mitochondrial function. This is the basis of the entire dark and light cycles that control all circadian cycles in higher animals. The key part of the cycles for biologic semiconductors is the incident light energy in UV light creates larger mechanical waves in tissues because these higher powered light frequencies alter and excite electrons in tissues and water. This energy has to be released from its excited state, and this causes a phase transition of water by red light. This light emitted by semiconductors at night can be a laser (August 2016 webinar). This monochromatic laser light emission optically effects our left handed proteins differently than D-glucose in the TCA cycle. In proteins, it can effect them by using other frequencies of light. Red light changes the density of water. If you look at the conduction band picture above you will density is the X axis controling semiconduction effieiciency. Water is one of the basic semiconductors in a cell and this explains why life cannot exist without it. To make light from an LED all one needs is a P/N junction and a current that is rectified. In the journal of Nature we now have proof that cells form lasers.
WHY IS DAYLIGHT KEY TO THE PRESENCE OF THE DC ELECTRIC CURRENT?
Sunrise has equal parts of red, green, and blue light frequency. Only blue and violet light is capable of of generating enough current to change the physics of organisms photoelectrically. No other part of the visible spectrum is strong enough to cause these phase transitions. AM sunlight provides a constant voltage for a short period of time and this results in no light production from a cells semiconductors initially in the AM. The electrons will begin to migrate into the band gap of collagen and will drift in the applied DC electric current field that AM sunlight provides. This eventually generates a DC electric current and emits some heat. Becker found this in his bone experiments. The small amount of heat liberated from the N-semiconductor is used to increase the size of the EZ and change the density of the band gap’s physics. This explains why collagen is the most common protein in humans and it is always surrounded by water. It also explains why DHA is always surrounded by water in the grey matter of the brain, too. Grey matter is grey because this is where mitochondrial density lies. If water was not present in CSF adjacent to the grey matter in our brain’s biologic semiconductors (DHA), over heating would result because temperature would rise too fast. This explains how halogenated anesthetics work to put humans to sleep in surgery. They allow massive influx of fluorine bound to the lipid soluble chemical into CSF to cause dielectric collapse. This massively disrupts the DC electric current because it causes semiconduction collapse, known as dielectric collapse in physics. Current concepts of electrobiologic controls owe more to developments in solid state physics than to biologic research. The feasibility of biasing the DC currents flow in the CNS to induce anesthesia or in the PNS to control pain syndrome also was demonstrated by Becker and Marino..
This small amount of heat made in semiconductive circuits increases the resistance of the semiconduction current in the other semiconductors on the surfaces of the brain.
The physics of band gap conduction: If you just apply a constant voltage to a piece of semiconductor, you will not get light out of it. All that will happen is that the electrons that are already in the conduction band will start drifting in the applied electric field, so you will get conduction and some heating. That small heat increases resistance to the current. This is why your iphone does not work in direct sunlight as its temperature rises. Now if you put it in the freezer immediately it will work extremely well. Why? Cooling the surroundings improves the conduction across the semiconductor. This is the basis of why cold thermogenesis is so effective during daytime hours when a small DC electric current is present in cells making them transparent to sunlight. In order to get effective recombination of electrons and holes (i.e. atoms that lack one electron), a PN-junction is needed, where the majority carriers in the N-region are electrons and the majority carriers in the P-region are holes lacking electrons making them more positive on a relative basis. The PN-junction forms a depletion region where the drifting electrons and holes neutralize each other (recombination by thermal diffusion) until the charge imbalance builds up a potential barrier that prevents further movement of charges. The device becomes non-conductive at that point (a diode at zero volts on its junction does not conduct except for a tiny thermal leakage current as mentioned above).
VARIATIONS OF SUNLIGHT DURING THE DAY ALTER BIOLOGIC SEMICONDUCTION
When nature applies an external voltage from the varying frequencies in sunlight on the junction that overcomes the potential difference in our surface semiconductors, then cells begin to move electrons from the N-region (collagen) to drift into the P-region and holes from the P-region will drift into the N-region. This situation readies the cell for the action of living. The process reverses at night when the UV light and then blue light slowly decreases at sunset. With the transition from dusk to night is how nature produces the necessary conditions for recombination of our semiconductive circuits to emit light because of the gradual presence of a reverse bias current. This situation allows for the conditions of existence for cells to produce significant amounts of light within cells below the surface for optical signaling. This is what occurs at night and stimulates the origins of sleep for regeneration. The presence of blue light in the environment absolutely destroys the reverse bias current and this is why sleep is destoyed by manmade light at night.

Now, before we proceed to explain the heart of sleep, we must understand why metal atoms conduct electricity to make a DC current for cells.
Every atom has an outer band of electrons, known as the valence band. In metals, the electrons from this valence band are not confined to the atom and are free to move throughout the metal lattice. It is this “sea of electrons” which makes electrical conduction possible. It is exactly the opposite in non-metals, where the electrons are held tightly. Electrical currents in metals and semiconductors are not equivalent. Semi-conductors act as non-metals at low temperatures – the electrons are trapped within the atom. As the temperature of the semi-conductor is increased, the electrons in the valence band gain sufficient energy to begin to escape from the confines of their atoms. As a result, in higher temperatures, a semi-conductor’s valence electrons become more free = conduction results and electrical resistivity decreases. We call the energy required for an electron to escape – the “band-gap” of a semi-conductor. (see pic above)
PHYSICS OF TRANSPARENCY
The higher the band-gap of a semi-conductor – more the energy is needed to convert it into a conductor. For example, the band-gap of Germanium is 0.67 eV, silicon at 1.1 eV. What can be deduced is that it takes a lower temperature to convert Germanium to a conductor than silicon. Carbon has a much lower band gap and this makes it special and this is why cells are made of collagen tubes. Collagen tubes are just like carbon nanotubes from a solid state perspective. Carbon nanotubes like collagen and microtubules have an electronic structure can be tuned by mechanical stretching and by magnetic fields. The mechanical stretching is provided by the Tensegrity system of the cell and varying magnetic fields are provided by mitochondria and polarized light.
The Kondo effect has been observed in quantum dot systems. All atoms are effectively quantum dots. Cells seem to like free radicals, phosphorus, sulfur, and the transition metals for their quantum dots. I think the choice was made by nature because these atoms are capable of making carbon based semiconduction transparent and opaque by varying magnetic fields in cells during night and day. If you look at solid state systems that use this effect, a quantum dot with at least one unpaired electron (free radicals) behaves as a magnetic impurity, and when the dot is coupled to a metallic conduction band (Fe-S cores in cytochromes), the conduction electrons can scatter off the quantum dot atoms. This changes the optical and magnetic transparency. Mitochondrial cytochromes have iron-sulfur couples. The iron and sulfur are quantum dots and can be programmed by light and magnetism. Iron is ferromagnetic. Sulfur is dimagnetic and acts as an impurity. Sulfur is a diamagnetic element, meaning that it has no unpaired electrons. This means it is not effected by a magnetic field. Since iron can be radically changed by electric and magnetic field to use the Kondo effect we would need an atom next to iron that very different quantum properties with magnetic field. Sulfur fits that need. The Kondo effect has been called a many-body phenomenon in condensed-matter physics which involves the interaction between a localized spin and free electrons. Mitochondria generate free radicals with unpaired spins and they contain iron-sulfur coupels which have a lot of electrons. The Kondo effect was discovered in metals containing small amounts of magnetic impurities. Sulfur is that impurity in mitochondria. Do we have proof of concept in sold state physics? Yes we do.
Zaric et al. (2004) studied the magneto-optical properties of small diameter carbon nanotubes (NT) in aqueous solution. An applied magnetic field aligned the NTs (parallel to the field) and shifted the energy of optical absorption and optical emission peaks. The large orbital magnetic moment of electrons in NTs have given researchers a powerful new tool to control the electronic structure of NTs. Nature has been using this for billions of years in cells. The tunnel transparency of p-n junction barriers can be tuned by using a magnetic field to modify the band-gap to change tunneling speeds and efficiency. This is the key mechanism of how % heteroplasmy manifests in a mitochondria. As % heteroplasmy rises tunneling speeds slow dramtically. When this occurs energy drops logrithmically. This is what changes nuclear gene expression. This has been the focus of Dr. Doug Wallace and me for ten years now. This use of optics-magnetic effects has been quite useful to nature for environmental seasonal sensing which effectively alters tunneling strengths in mitochondria during different environments and seasons. This is how nature fine tunes the energy levels of electrons in the mitochondria and in our N-type semiconductors like collagen and microtubules in cells.

The non-conductivity of a semiconductors is due to the charge carriers (both positive and negative) becoming immobile. As we know heating an object gives its particles kinetic energy as they vibrate more. A gain in kinetic energy by altering light frequencies daily(UV light) means they vibrate more rapidly making them mobile. This Kondo effect helps mitochondria sense day and night as well because it affect the magnetic field strength in mitochondria. The electrons in the atoms (that are quantum dots; Fe-S) move away from the nucleus and can be easily removed by small voltage applied across the band gap. In mitochondria the voltage is quite large. So, heating makes the charge carriers more mobile. As charges move, the wave effects of light become more prominent because electromagnetic waves originate always by the movement of charges.
LIGHT IN MITOCHONDRIA
I’ve now established that mitochondria are loaded with semiconductors and LED’s. LED’s create light by electroluminescence in a semiconductor material. Electroluminescence is the phenomenon of a material emitting light when electric current or an electric field is passed through it – this happens when electrons are sent through the material and fill electron holes. An electron hole exists where an atom lacks electrons (negatively charged) and therefore has a positive charge. For example in bone, biologic semiconductor materials like collagen (carbon NT) and apatite are “doped” by two copper ions (quantum dot) to create and control the number of electron holes. Copper ions are transition metal that biology uses in different tissues where collagen is the key N type semiconductor. Doping is the addition of others elements to the semiconductor material to change its physical and optical properties.
By doping a semiconductor with a transition metal you can make two separate types of semiconductors in the same crystal or quasicrystal. Collagen is a flexible piezoelectric crystal and water is a liquid quasicrystal that is anisotropic. Anisotropy is just another fancy word for making order from the atomic chaos naturally found. You can use many things to improve anisotropic arrangements, like transition metals, that allow biologic semiconductors to become ferromagnetic or diamagnetic to become more ordered. They also can change the frequency of light semiconductors emit. Static fields affect organisms by their ability to orientate magnetically sensitive molecules and by deflecting endogenous electrical currents. This is how a magnetico sleep pad can exert control for defective cells. Some molecules can orientate themselves in a magnetic field. Water and proteins can do this especially when transition metals are part of their lattice. Some molecules can orientate themselves in a magnetic field. In semiconduction, which QED rules control, electron pairs have spins that oppose one another.
As a consequence of their electron spins, electrons behave as though they are miniscule bar magnets each possessing a magnetic moment, called the Bohr magneton, and is of the order of 10−23 J/Tesla. A complete electron shell always contains pairs of electrons with opposite spins. In the transition metals, iron, cobalt, copper, and nickel, there are unpaired electrons, not only in the outer shell but also in an inner D shell. In iron, for instance, five of the six electrons in the n = 3, l = 2 subshell have parallel spins, so that iron atoms have appreciable magnetic moments and are referred to as ferromagnetic. This is what makes these metals special for biology and why they are used in proteins of the cytochromes and blood plasma. These electrons become able to do some amazing things when they can freely float in a semiconductor lattice when sunlight is introduced to these electrons photoelectrically. Water and collagen are that lattice that is life’s stage. The Kondo effect is just another non-linear effect of light that life uses.
Atoms and molecules with fewer unpaired electrons do not form electron domains, they simply become paramagnetic or diamagnetic. A paramagnetic atom or molecule has a permanent dipole moment so it is drawn to magnetic fields. Oxygen is biology’s best example and it is the temrinal electron acceptor in mitochondria for its paramagnetism. This means inside our tissues, oxygen acts like a magnet all the time, and this is why it is drawn to mitochondriawhere large magnetic fields are generated. Oxygen, therefore, tends to align itself in the direction of the applied field it finds itself in. A diamagnetic atom or molecule, on the other hand, has no permanent dipole moment. This means it can only be magnetic when the field allows for it. This si why sulfur is next to iron in cytochromes. It remains silent until the Kondo effect varies the magnetic field during night and day. Sulfur normally it is not magnetic therefore it is not drawn to magnetic fields until something changes its electron spin or configuration.
HOW DOES THIS SCIENCE AFFECT CELL MEMBRANES WHICH CONNECT TO MITOCHONDRIA?
When an applied magnetic field is added to the new environment of these atoms, as it is in a mitochondria on a diurnal basis, this immediately affects the orbital motion of the electrons in such a way as to produce a magnetic moment in the opposite direction to the applied magnetic field. This is how free radicals are made at cytochrome proteins. All free radicals have unpaired electrons spins. The effect of static magnetic fields on biologic semiconductors, like Earth’s geomagnetic field during day and night, affects the permeability of lipid vesicles on cell membranes which helps to summate all of diamagnetic alignments of large molecular aggregates to the external magnetic field. This is usually seen to occur near eukaryotic cell membranes which are adjacent to EZ water. This is considered an interfascial zone for water because it is at a transition between the cell membrane and EZ water. These rapid changes occur at various temperatures for each chemical footprint. This is why the isomerization step for sulfated Vitamin D3 synthesis in the skin is increased by UV light heating up the epidermis. I talked to you about phase transition temperatures when we spoke about water in the past. A phase transition in water is when it goes from a gas to liquid state or vice versa. The transition from bulk water to EZ water is another example of a phase transition.

In physics, all transition states are linked to TEMPERATURE changes. The temperature changes alter the size and shape of molecules. This is the basis of how thermodynamic laws operate universally. As things get smaller they are more energy efficient. As things get larger they become less energy efficient. Sunlight is capapble of changing temperature of things so it is capable of altering the thermodynamics of proteins, water, and lipids. So as one gets more obese, respiratory proteins enlarge because magnetic flux decreases, water is lost, dehydration lowers the dielectric constant of water, this lowers the ability to make sulfated Vitamin D3 in skin, and excessive amounts of light is emitted by cells because of a loss of magnetic flux in mitochondria. Most biologic proteins, like collagen, have transition metals in them. This is certainly true in the mitochondria. These atoms have the innate atomic ability to create order using sunlight; they do not need any other form of energy to orgnaism tissue lattice’s. Geomagnetism is stronger during night time than day time. It turns out all semiconductors exhibit an “effective diamagnetism” when they experience a changing magnetic field. This means due to Earth’s normal small varying diurnal magnetic field, fluxes and currents can change, and as such, these changes can be transferred directly to biologic semiconductors in cells.
WHAT IS LIFE? SLOWING LIGHT USING SEMICONDUCTORS.
When light’s speed is constant at 186,000 mph hour time is absent. If time is absent life cannot exist. So what is a cell’s main goal? It is a playground for photons to capture light and use it; and this allows time to emerge. We use light at night when we sleep to regenerate our tissues optically so we can capture more light to slwo time to increase longevity. As time emerges, then and only then life becomes possible. This is why good sleep is associated with optimal logevity and performance. But when light slows as it interacts with matter, time manifests only because it slows down from it constant speed. With the advent and emergence of time, these energy cycles become coupled to light and dark periods. This is why circadian biology is fundamental to living things. This coupling of energy cycles permeate many aspects of Earth’s ecosystem such as thermohaline, climate, or vortices in weather. The small varying magnetic field becomes a natural resonant coupler of many different cycles naturally without using or needing any other energy source. This is the basis of the Rayleigh Benard convection cells in water I mentioned in the RPE in the Ubiquitination series whn discussing the photoreceptor function of the eye. This convection heat is release deep inside of our tissues from mitochondria and as it cools toward our surfaces these Rayleigh Benard convection cells form spontaneously to provide energy indefinitiely for no cost. These coupled systems use these metal ions to continue to bring order to a cell by reducing its entropy or randomness. This reduction of entropy allows this red light (heat from mitochondria or semiconductors) to be captured by the heat sink of EZ water, As a result, light is further slowed down by the EZ and this results in a higher density of the EZ water. A higher density means that the EZ water will have more electrons and this is why the EZ carries a net negative charge and excludes protons.
WHY IS MRI A POWERFUL REDOX TOOL FOR LIGHT ASSIMILATION?
Because water can change its own physical characteristics just using sunlight’s spectral variantions during the day and its complex non linear effects, allows water to be the ultimate chamelon in cell and helps explain why water is anisotropic. The anisotropic effect allows neurosurgeons to use water diffusion imaging for diagnosis. EZ water makes MRI sensitive to the underlying tissue microstructure. This provides physicians a unique method of assessing the orientation and integrity of tissues filled with semiconductors, which offer the trained eye to be quite useful in assessing a number of patholigic disorders linked to altered light signals.
Water is an anisotropic quasicrystal and sunlight’s frequencies and energy orders its crystaline form. Many studies show that the cell water forms bilayers to form a dodecagonal quasicrystal, as well as two bilayer crystals, one tiled exclusively by pentagonal rings. Quasicrystals and liquid crystals have structures with long-range order but without periodicity, have now been reported in the literature (EZ). Liquid crystals are relatively easily aligned with electric and magnetic fields, which is the basis of the liquid crystal display screens that come with modern TV’s, iphones and ipads, watches, laptop computers and every computer game made today. Cells do the same thing using water and their cell membranes. Pure samples of lipid membrane are known to align themselves in an external magnetic fields. All proteins in living things are surrounded by water. Proteins are also diamagnetic due to the planar peptide bonds, which, in the case of the α-helix (collagen), in particular, are all aligned along the axis, and this will give rise to substantial diamagnetic anisotropy that further orders a cell when it is in sunlight. The impurities of copper ions in collagen change its physical abilities as magnetic fields vary.
These physical features found in EZ water demonstrates that these novel phases are intrinsically favored in living tissue and further suggests that these structures are very relevant in biology in DNA, microtubules, collagen fibers, and in intermitochondrial junctions (IMJ’s). They not only for confined water, but also for the wetting and properties of water at interfaces with organelles and cell membranes.
SUMMARY
Mitochondria are surrounded on all side by EZ water in its MINOS layer. This makes boundaries where is an EZ is present a very special biologic semiconductor with multiple abilities to change respiratory proteins geometry while using a DC electric current and light emitter by altering temperature changes and magnetic flux that occur.
The boundary between the two types is called a p-n junction is an interfascial space for EZ water. The junction only allows current to pass through it one way, this is why they are used as diodes. LED’s are made using p-n junctions. As electrons pass through one crystal to the other they fill electron holes. They emit monochromatic photons (light). This is how a semiconductor laser works in a cell to create consciousnes, memory, and life.

CITES:
See hyperlinks in blog
- http://www.ncbi.nlm.nih.gov/pubmed/20969412
- Sydney Leach – 2012 Physical Principles and Techniques of Protein Chemistry. http://www.i-sis.org.uk/SuperconductingQuantumCoherentWaterinNanospace.php

